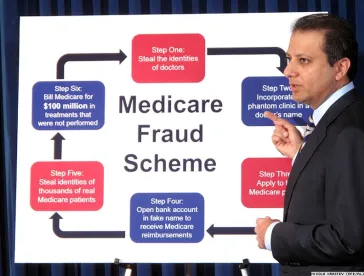
In one of the largest ever health care fraud schemes charged by the Department of Justice, cancer genetic testing and telemedicine are at the center of the scheme. On September 27, the DOJ announced charges against 35 individuals for an alleged $2.1 billion in fraudulent Medicare billing for cancer genetic tests. The purported scheme involved kickbacks that cancer genetic testing laboratories paid to medical professionals working with telemedicine companies in exchange for ordering tests. The tests were allegedly not provided, medically unnecessary or ordered with little or no established physician-patient relationship.
The charged defendants include individuals holding various roles throughout the scheme: physicians, company owners, CEOs, CFOs, marketers and others.
Potential liability for those involved extends beyond criminal liability. The CMS’ Center for Program Integrity took adverse administrative action against certain cancer genetic testing companies and professionals that submitted more than $1.7 billion in Medicare claims.
Separate from the genetic testing enforcement action, the DOJ recently announced several other large-scale fraud takedowns:
-
Also on September 27, the DOJ announced charges against 53 individuals in Michigan, Illinois and Minnesota regarding an alleged $250 million in illegitimate Medicare and Medicaid claims. The underlying fraud schemes included alleged kickback schemes and the submission of claims for medically unnecessary services or services never provided.
-
On September 26, the DOJ announced charges against 48 individuals in Connecticut, D.C., New Jersey, New York and Pennsylvania, including 15 physicians or medical professionals, primarily for their roles in diverting opioids or kickback schemes involving telemedicine and DME companies, with $160 million of fraudulent claims at issue.
-
On September 25, the DOJ announced charges against 67 individuals in Florida and Georgia for conduct resulting in more than $160 million in alleged fraudulent claims. The purported conduct included kickback schemes with telemedicine companies and laboratories. The DOJ also charged 33 individuals for various alleged fraud schemes, including obtaining controlled substances by fraud, resulting in more than $515 million in fraudulent billings.
While we predicted the Government would increase its enforcement efforts related to genetic testing in our August update, the DOJ’s most recent actions support two broad areas of continued focus. First, telemedicine remains an area ripe for investigation and enforcement. Second, the Government does not overlook the role of individuals involved in the alleged wronging – including that of physicians – and will consider criminal liability for health care fraud.
Increased enforcement actions within the telemedicine and genetic testing industries serves as a reminder for those working in these spaces to proactively monitor compliance with health care laws, including the Anti-Kickback Statute and False Claims Act, and ensure relationships they have with others remain legitimate and compliant. In doing so, companies and professionals will be well-served by consulting with counsel.



 />i
/>i
