On Nov. 4, 2021, CMS published an Omnibus COVID-19 Health Care Staff Vaccination interim final rule, requiring Medicare providers and some suppliers to ensure their staff are fully vaccinated by January 4, 2022 (CMS Mandate). The CMS Mandate covers a wide swath of personnel at almost all Medicare providers and some suppliers—all of which are subject to the Medicare certification requirements
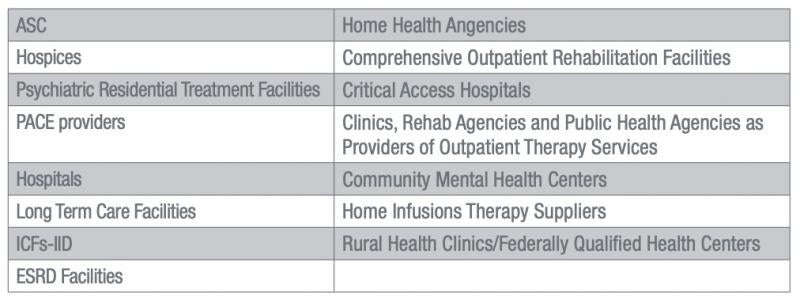
Providers and Suppliers Covered
What is Required
-
By December 5, 2021, all providers and suppliers subject to this rule must have:
-
A process or plan for vaccinating all eligible staff.
-
1st Dose or One Dose Vaccine by Dec. 5, 2021. • Full vaccination by January 4, 2022.
-
A process or plan for providing exemptions and accommodations for those who are exempt.
-
A process or plan for tracking and documenting staff vaccinations.
-
Tracking can be in any form, including use of NHSN vaccination tracking tool available to the public.
-
Proof of vaccine documentation may be the CDC vaccination record card, documentation from a health care provider or electronic health record or the State immunization information system record. All records must be kept confidential and stored separately from an employer’s personnel files.
How Will CMS Enforce this Mandate?
The CMS Mandate does not establish any additional reporting requirements, even for facilities subject to quality reporting measures.
Enforcement will be based on
-
Conditions of Participation/Conditions for Coverage, so the CMS Mandate will be enforced as other COPs/CfCs through the survey process, inclusive of accreditation surveys.
-
Deficiencies, Plan of Correction, possible CMPs, possible termination.
-
The CMS Mandate pre-empts any state law
-
CMS expects to issue interpretive survey guidelines, along with training for state surveyors (record review, staff interviews, how to cite noncompliance).
Who is Included in “Staff” Required to be Vaccinated?
-
Staff are employees of the provider or supplier, licensed practitioners, students, trainees, and volunteers, and any individuals who provide care, treatment, or other services for the provider or supplier under contract or other arrangement.
-
Staff includes non-clinical personnel such as administrative personnel, housekeeping, food service and volunteer and other fiduciary board members.
-
Staff includes any individuals who perform their duties at any site of care, including independent contractors (i.e. medical staff).
-
The CMS Mandate does not apply to staff who exclusively provide telehealth, telemedicine, or support services who do not have any direct contact with patients and other staff and work outside the site of care.
-
The CMS Mandate does not apply to individual vendors who provide infrequent and ad-hoc non-health care services (for instance a vendor brought in to inspect an elevator, delivery or other repair personnel).
Who is Exempt?
-
Each provider or supplier must offer “a process by which staff may request an exemption from the staff COVID-19 vaccination requirements based on the applicable Federal law.”
-
Exemptions may be granted due to religious beliefs or under applicable Federal Law.
-
If a medical exemption is requested, then the provider or supplier must obtain documentation that confirms a clinical contraindication to the COVID 19 vaccines. This must be signed and dated by a clinical practitioner who is acting within their state scope of practice, but it may not be signed by the person seeking the exemption. The documentation must set forth recognized clinical reasons for the contraindications and a statement from the practitioner recommending that the staff member be exempted.
-
CMS takes the position that the requirements of the Interim Final Rule preempt any state or local laws that may allow for broader exemptions than those permitted under federal law.
What is Fully Vaccinated?
-
Johnson & Johnson = 14 days after receipt of vaccine.
-
Pfizer-BioNTech or Moderna = 14 days after receipt of the second of the two-dose primary vaccination series.
No booster shots are required to comply with the CMS Mandate.
In the event a staff member receives a vaccine outside the U.S. that is neither FDA approved nor authorized, CMS defers to the CDC guidance which generally advises that individuals who have completed a vaccine series listed for emergency use by the WHO should not also obtain another FDA-approved or authorized series in the U.S.
How Does the CMS Mandate Work with the OSHA and other Federal Vaccine Requirements
Facilities subject to the CMS Mandate must comply with the CMS rule first. If a health care provider or supplier is not subject to the CMS Mandate, then the Executive Order on Ensuring Adequate COVID Safety Protocols for Federal Contractors or the OSHA COVID-19 Healthcare Emergency Temporary Standard applies. If none of the above rules apply (CMS Mandate, Executive Order or OSHA ETS), then employers with more than 100 employees are subject to the OSHA Employer Emergency Temporary Standard.




 />i
/>i

