For decades, FDA’s Center for Devices and Radiological Health (CDRH) has been recognizing standards that can be referenced in premarket medical device submissions.
Congress broadly directed federal agencies to begin relying on standards in 1996, through the National Technology Transfer and Advancement Act, but the informal practice dates back to the 1970s. Congress specifically directed FDA to begin using standards for medical device submissions through the Food and Drug Administration Modernization Act of 1997 (FDAMA).
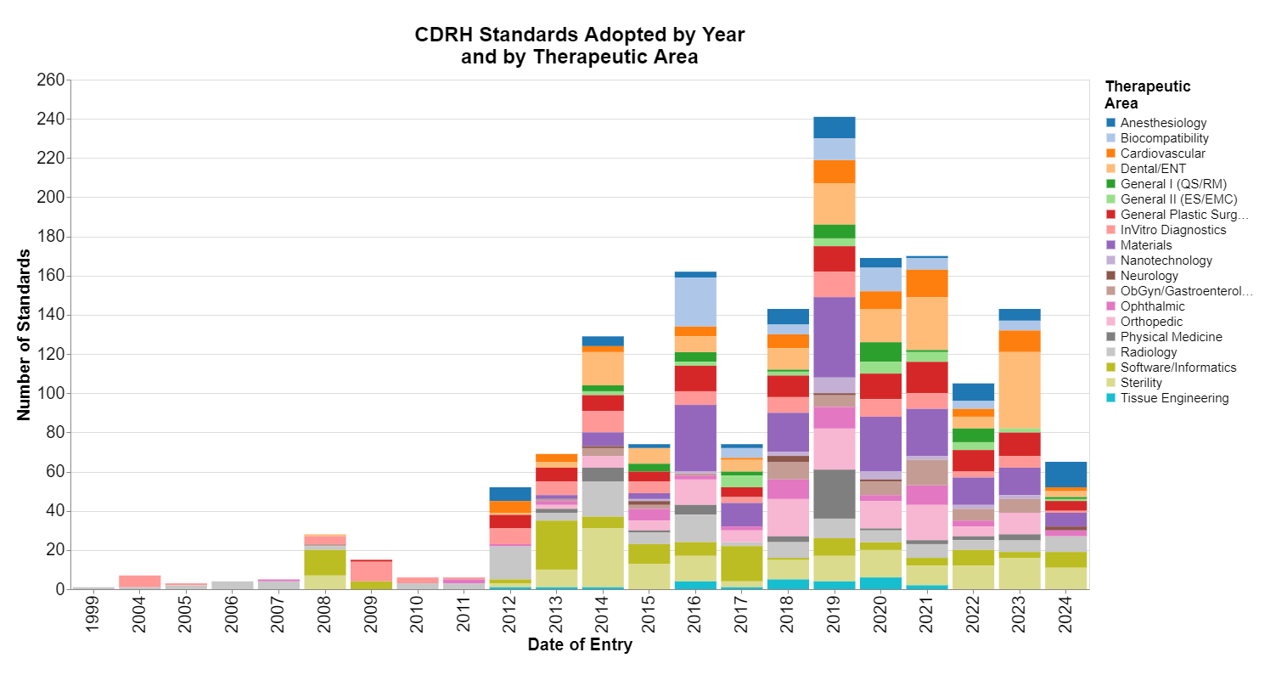
Being a curious person, I wanted to see what FDA has done with that authority by looking at the CDRH database for Recognized Consensus Standards: Medical Devices. My main takeaway is that CDRH is not yet investing enough time and energy in recognizing standards that support digital health and AI.
Findings
I downloaded the data set on September 20, 2024, and looked when standards were recognized by FDA and to which therapeutic or functional areas they related.
As always, I used calendar years because it’s easier for us nongovernmental people to understand, but that also means that 2024 was a partial year.
Methodology
This month, the methodology was straightforward. The data set identifies the therapeutic areas to which they relate, and the date the standard was entered into the database.
My reference to “therapeutic area” may be the wrong word choice, but FDA describes it as “specialty task group area,” which I didn’t think was very meaningful. These are not all the traditional Advisory Committee areas, but rather include some descriptions such as “materials”, “nanotechnologies” and “software.” That makes sense because certain standards will be horizontal standards applicable to many therapeutic areas.
Interpretation
This database is dynamic in that both standards are added, but also standards are deleted when they are no longer recognized by FDA. I came to appreciate this because the above chart was created using a data set that I downloaded on September 20, 2024 and that data set had 1,671 standards in it. A few weeks later, on October 10, 2024, I downloaded the data set again and at that point it only had 1,552 standards. The newer data set had two new standards were entered in the beginning of October. I stuck with the larger data set from September, but my point is that looking at those early years is notindicative of the recognition activity that occurred at that time because presumably the standards FDA has sunset are more likely from the earlier time periods.
This means I can’t really infer a whole lot regarding adoption trends because I’m not able from this data to control for standards that are deleted. To do that, I would need to go through the Federal Register to look at the raw data on standardized recognition rather than focusing on the database. There have been 62 FR notices published over the last 25 years recognizing standards, and many of the changes are simply to withdraw an older version and replace it with a newer one. To be honest, it just seemed like too much work. So I just stuck with the FDA database and I won’t make inferences regarding trends from the earlier time periods.
In reading any chart such as this, my initial reaction is always to look at the first part of this decade as the so-called COVID years. I don’t know how much COVID really delayed standards development work, because I imagine quite a bit of preceded via Zoom. But it’s also easy to believe that FDA was preoccupied with its emergency response to the pandemic, and so actual recognition of standards may have been delayed.
I am a bit surprised that we have apparently some radiology and orthopedic standards still in use that are more than 20 years old. I’m surprised they haven’t been updated, but that also suggests that maybe some technology areas simply haven’t evolved much. I guess I’m more surprised about radiology than I am about orthopedics. I thought most aspects of radiology have changed pretty radically.
On the disappointing side, I was hoping that I would see a general growth in standards related to software because of the advent of digital health and AI, but these data really don’t suggest any such trend.
On the plus side, I’m glad to see that materials are getting a lot of focus, as it’s my impression that we are in the middle of a materials revolution of sorts. The development of new standards is facilitating the introduction of these exciting new materials.
The use of standards
FDAMA amended Section 514(c) to state, in part, that FDA “shall, by publication in the Federal Register . . . recognize all or part of an appropriate standard established by a nationally or internationally recognized standard development organization for which a person may submit a declaration of conformity in order to meet a premarket submission requirement or other requirement,” 21 U.S.C. § 360d(c)(1)(A).
FDA’s current guidance on Appropriate Use of Voluntary Consensus Standards in Premarket Submissions for Medical Devices was finalized in 2018, and replaced a September 17, 2007 document which replaced a March 12, 2000 document. Use of consensus standards to meet premarket submission requirements can help facilitate the premarket review process and is not limited to use in Abbreviated Premarket Notifications (510(k)s), but can also be used for any 510(k), De Novo request, Investigational Device Exemption (IDE) application, Premarket Approval (PMA) application, and others.
Conclusion
While the general development and use of standards at CDRH seems appropriate, my main takeaways that the center is not yet investing enough time and energy in recognizing standards to support digital health and the adoption of AI. That’s disappointing.



 />i
/>i