The cannabis industry is booming, with more and more states expanding the legal cannabis options available to the public.
The Current State of FDA Regulation of Cannabis Products
The FDA’s mission is to protect public health by ensuring the safety, efficacy, and security of multiple product categories including drugs, medical devices, foods, and cosmetics. The agency’s regulatory authority also encompasses products in these categories that are made from cannabis or cannabis-related compounds.
“Prior to COVID, many people thought of the FDA as a single entity,” Hatcher said. In fact, it is composed of six separate centers—the two that primarily regulate cannabis products are the Center for Food Safety & Nutrition (CFSAN) and the Center for Drug Evaluation & Research (CDER).
Other federal agencies also regulate cannabis and cannabis-derived products, including the U.S. Department of Agriculture (USDA), the Drug Enforcement Agency (DEA), the National Institute on Drug Abuse (NIDA), and the Federal Trade Commission (FTC). State-level regulatory agencies, such as state Departments of Agriculture, also regulate the cannabis industry. In addition, the National Animal Supplement Council (NASC) and Association of American Feed Control Officials (AAFCO) work with the FDA to regulate animal foods, including those containing cannabis-derived products.
“Hemp” and “marijuana” actually belong to the same species, Cannabis sativa, but are legally distinguished based on their delta-9 tetrahydrocannabinol, or Δ9-THC content. Hemp contains 0.3% or less Δ9-THC, while anything above that threshold is classified as marijuana.
To date, the FDA has approved four cannabis drug products, mainly focused on CBD and THC. They are:
-
Marinol used to treat nausea in cancer chemotherapy patients and weight loss in AIDS patients (Schedule III).
-
Syndros, used for similar purposes (Schedule II).
-
Cesamet, another nausea drug for cancer chemotherapy patients (schedule II).
-
Epidiolex, a CDB-based medicine used to treat pediatric seizure disorders, Dravet syndrome and Lennox-Gastaut Syndrome. Unlike the other three drugs, Epidiolex is no longer classified as a controlled substance.
No cannabis-based animal or food products have been approved by the FDA. It’s important to note that while cosmetics do not need to be reviewed by the FDA prior to marketing, it is the responsibility of those marketing a cosmetic product to ensure the safety of the product. No ingredient – including CBD – can be used in a cosmetic if it causes the product to be adulterated or misbranded in any way.
The 2018 Farm Bill preserved FDA’s regulatory authority over hemp, gave the USDA authority over the production of hemp, and removed hemp from the definition of “marijuana.”
“There are a few specific products that the FDA has acknowledged to be “generally recognized as safe” or GRAS for use in human food,” Hatcher said. These are hemp seed oil, hemp protein powder, and hulled hemp seed.
However, there are numerous hemp-derived CBD products on the market that are not approved by FDA for human or veterinary use. So, in May 2019, the FDA held a public hearing on regulated cannabis products to help inform oversight of these products. The event drew more than 100 speakers, and more than 4,500 public comments have been submitted. However, Hatcher said that no new CBD-related regulations have yet resulted from this hearing.
Regulatory Trends for Cannabinoid Products
Despite the lack of any new regulatory policies, public comments made by FDA officials may provide some clues to future regulatory trends.
In July 2019, Principal Deputy Commissioner Dr. Amy Abernethy testified before the U.S. Senate. She said the agency recognizes the need for “regulatory clarity, particularly given the significant public interest in hemp products and CBD in particular.” But she added that manufacturers selling “any CBD food or purported dietary supplement products in interstate commerce do so in violation of the FDCA (federal Food, Drug and Cosmetic Act).” Dr. Abernethy said the FDA is exploring the possibility of new legal pathways to bring CBD products to the market.
And in a November 2020 presentation, Dr. Abernethy said “the science of CBD and other cannabinoids has become an FDA priority…There’s a mounting need to consolidate what we know about these products and to clearly communicate knowledge gaps to the public.”
The FDA’s renewed interest includes establishing a CBD Policy Working Group, which has released a few guidance documents, but no new FDA regulations. Last year, former FDA Commissioner Stephen Hahn reported to Congress on CBD regulation and enforcement, but his comments only reiterated the status quo and didn’t give much indication of where the FDA could be heading in this field.
“With the passage of the 2018 Farm Bill, you saw this explosive growth of the category and so many products entering the market. But not all CBD products are created equal in terms of quality standards. Many products and brands do a great job with that and others do not,” Brown said.
She said it is important for CBD brands not to make unjustified claims about their products and their health benefits. “We follow that guidance and let our clients know what they need to put on their packaging,” she said. “We know these products do have benefits, but you have to be clear and careful about how you do that.”
"Not all CBD products are created equal in terms of quality standards. Many products and brands do a great job with that and others do not."
NICOLE BROWN, CHIEF INNOVATION OFFICER, OPEN BOOK EXTRACTS
The industry is starting to commission its own research to submit to the FDA in order to fill knowledge gaps about these products.
“The FDA wants to see good science and it wants to see a commitment to quality,” Hatcher said.
Enforcement Trends in CBD & Cannabis Regulations
Three major federal agencies handle the bulk of CBD and cannabis regulatory enforcement.
-
The FDA can seize unregulated products, file injunctions and impose both criminal and civil penalties.
-
Likewise, the DEA can levy criminal and civil penalties against violators.
-
The FTC can freeze assets, issue temporary restraining orders and injunctions, and pursue litigation to enforce its regulatory standards.
Recently, the FTC conducted Operation CBDeceit, which targeted CBD companies making overt claims that their products could treat specific diseases. The FDA has sent out nearly 20 warning letters to companies related to COVID-19 claims and cannabis-derived products.
So what are the pathways for FDA authorization of cannabis and cannabis-derived products?
Under the FD&C Act, any product, including a cannabis product (hemp or otherwise), that is marketed with a claim of therapeutic benefit, or with any other disease claim, is considered to be a drug and, thus, must be approved by the FDA.
“The FDA does have some expedited and priority review programs for which your product may qualify,” Hatcher said. But, products still must meet the same high regulatory standards.
"The FDA wants to see good science and it wants to see a commitment to quality."
DR. HEATHER HATCHER, REGULATORY SCIENTIST, WOMBLE BOND DICKINSON
Pathways for FDA Authorization
The pathways for FDA authorization depend on whether the product is intended as a drug or as a dietary supplement.
“For a drug, the pathway includes a pre-IND (Investigational New Drug Application) meeting with the FDA, then pre-clinical testing, submitting an IND and then, clinical trials,” Hatcher said. Once these steps have been completed, the company may submit a New Drug Application (NDA) to the Center for Drug Evaluation & Research.
“This is a costly procedure, so I would encourage you to reach out to the agency early and to seek legal and regulatory assistance,” she said.
For dietary supplements, companies prepare and submit a New Dietary Ingredient Notification. This must include identity information on the new ingredient, history of use and evidence of safety. The FDA then reviews the application for a 75-day period.
The FDA’s Center for Veterinary Medicine is responsible for evaluating animal drugs, which includes looking at how this product may impact the safety of the human food chain.
“The animal product sector, when it comes to CBD products, has been growing quite rapidly,” Brown said. “These products are highly regulated, and the NASC has a list on its website of what products are approved. Cannabinoids can be beneficial for pets as well as humans—but we want it done the right way.”
Other Cannabinoids—And Latest on Δ8-THC
There are more than 480 naturally occurring components found in the Cannabis sativa plant—more than 100 of these are classified as cannabinoids. “There are a number of other cannabinoids other than CBD and THC that may be of economic interest,” Vaders said. Anecdotally, many of these are reported to have therapeutic purposes and are the subject of research.
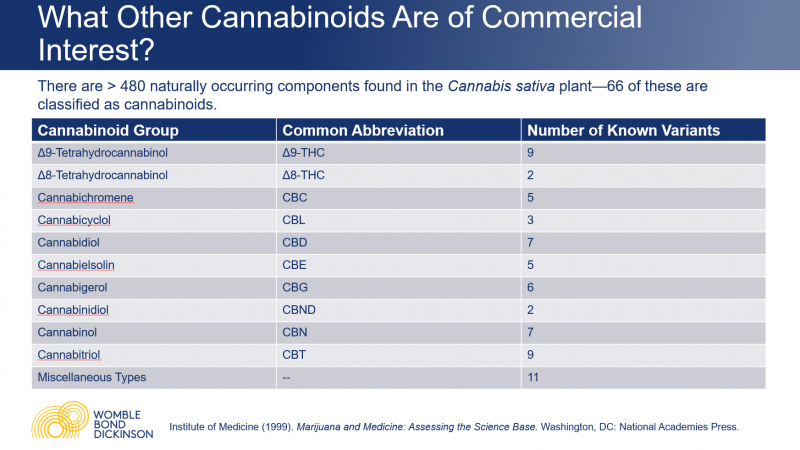
The Δ8-THC cannabinoid is of particular economic interest at the moment, because it is far more profitable than the CBD from which it is synthesized. “The ability to synthesize it from hemp-derived CBD is what has given rise to the legal gray area that Δ8-THC is currently living in,” Vaders said. “It’s unclear whether it falls under the definition of hemp, and may thereby avoid scheduling under the Controlled Substances Act.”
Brown said the type of process used to create Δ8-THC is problematic, resulting in products of dubious quality and safety.
“When you are talking about Δ8-THC production, you aren’t talking about a process that is taking place in a lab, conducted by Ph.D. scientists,” she said. “The quality is low and impurities that exist can be dangerous and toxic.” However, products are getting into consumers’ hands, despite these dangers, she said.
“It’s a problem we hope is addressed quickly,” Brown said. “We don’t believe the intention of the Farm Bill is for products like Δ8-THC to proliferate in the market.”
The FDA and CDC have warned consumers about the dangers of Δ8-THC, noting that it has not been evaluated or approved by the FDA and can produce psychoactive and intoxicating effects, as well as may contain potentially harmful chemicals and contaminants.
Another cannabinoid of interest is Δ9-THCP, a naturally occurring compound that is approximately 30 times more potent than Δ9-THC. There are also THC-O-acetate products said to be 300 times more potent. But again, Brown said these cannabinoids have not been vetted and approved for safe use.
Vaders emphasized that it is crucial to, “Know what is in your product before you put it on the market.”
“Know what is in your product before you put it on the market.”
MARK VADERS, ATTORNEY, WOMBLE BOND DICKINSON
So can these new cannabinoids be legally marketed? A new drug application is one pathway, but that route requires a great deal of research and cost.
A company also could try to market such products as a food or new dietary ingredient. Vaders noted that the FDA recently rejected two NDI applications that sought clearance to market a full-spectrum hemp extract containing CBD. The FDA rejected those applications, saying that CBD is already present in an approved drug product. Under the FDA’s Drug Exclusion Rule, the FDA generally does not allow approved drugs to be used as dietary supplements.
The FDA also cited vague, insufficient data in rejecting the two NDI applications.
“The bottom line is the FDA is focused on product safety. They want to make sure the products aren’t going to harm people, even at the highest levels of use,” Vaders said.
“This goes back to being really thorough with the science we’re collecting and putting out in the market,” Brown said. “The FDA isn’t closing the door on these products, but they’re saying, ‘We need to see more.” Interested parties can go to ClinicalTrials.gov to see what research exists for a wide range of products, including cannabinoids.
Beyond the FDA, the DEA “has been making noises around whether they view compounds like Δ8-THC as synthetically derived,” Vaders said. The DEA released a 2020 interim final rule noting that the 2018 Farm bill limits the control of tetrahydrocannabinol. However, the agency believes that all synthetic THC is still classified as a Schedule 1 controlled substance.
Also, under Federal Analogue Act, controlled substance analogues (CSAs) are treated the same as the controlled substance if the chemical structure is “substantially similar” and its CNS effects “substantially similar to or greater than” the actual controlled substance.
“Obviously, this has implications for psychoactive products such as Δ8-THC, THCP and THCO,” Vaders said.
Points to Consider
-
Know what is in your products
-
Review product claims
-
Monitor all social media platforms for claims & links
-
Follow cGMP
-
When in doubt, hire an experienced legal/regulatory consultant
-
Review the FDA’s recent Warning Letters.




 />i
/>i
