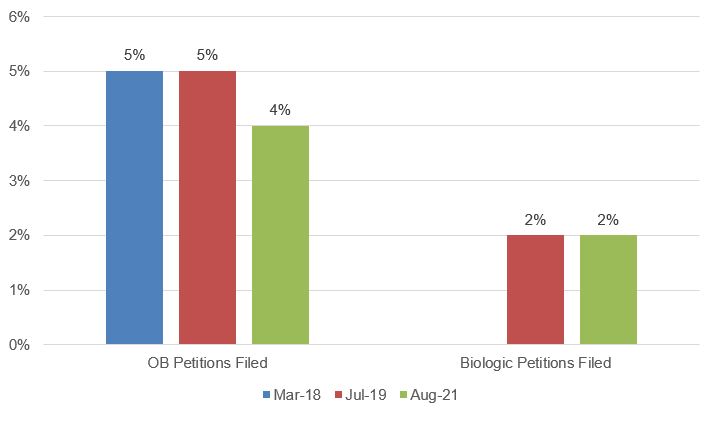
The USPTO released an update to its PTAB Orange Book Patent/Biologic Patent Study on August 11, 2021. The initial study was released on March 13, 2018, and the second was released on July 18, 2019. This report is the third issued by the USPTO to provide data on Orange Book-listed patents challenged in AIA post-grant proceedings, and the second with data on biologic patents. The data from these reports show that petitions challenging Orange Book-listed and biologic patents remain a small percentage of the overall number of petitions filed (see Fig. 1). These low percentages possibly reflect the perceived strength of these patents or parties’ preferences for adjudicating patentability/validity in other forums, particularly in light of the special Hatch-Waxman and BPCIA litigation procedures.
Fig. 1. OB and Biologic Petitions Filed as Percentage of Total Petitions Filed[1]
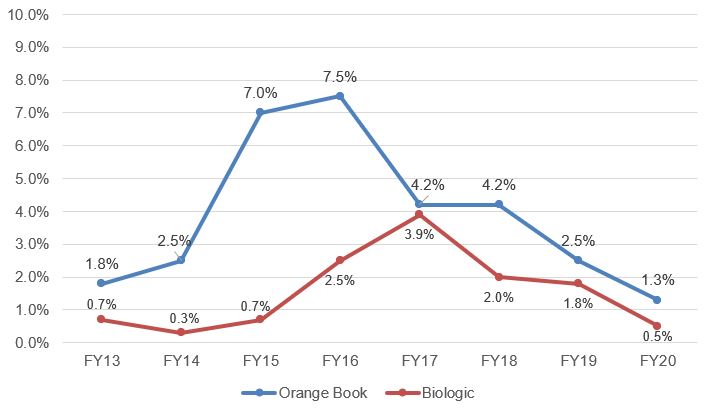
In addition, the data revealed that after reaching peaks in FY16 and FY17, respectively, the percentage of petitions challenging Orange Book-listed and biologic patents has been steadily declining (see Fig. 2). While this trend could reflect the strength of the remaining patents after initial challenges to the “low hanging fruit,” the drop in petitions could also be due to decreasing institution rates (discussed below), creating a seemingly less favorable forum for petitioners.
Fig. 2. Trend in OB and Biologic Petitions Filed as Percentage of Total Petitions Filed[2]
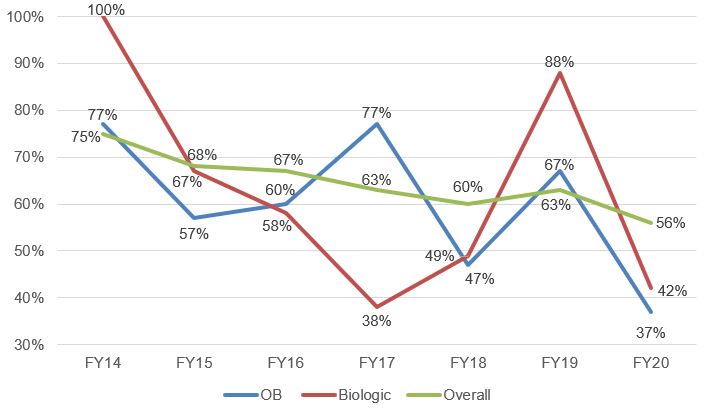
Institution rates on petitions challenging Orange Book-listed and biologic patents, like overall institution rates, have been dropping since 2013, though both have fluctuated more dramatically over time than overall institution rates. In Fig. 3 below, the overall rate shown by the green line has far less fluctuation than the red and blue lines representing Orange Book-listed patents and biologic patents, respectively. It must be remembered, though, that the data sample for OB-listed and biologic patents is much smaller than that for IPR petitions overall. Therefore, the data is more volatile. Bearing that in mind, the greater variation in Orange Book-listed and biologic petitions is of interest. For example, in FY17, when the overall institution rate was 63%, the institution rate for petitions challenging Orange Book-listed patents was 77%, while the institution rate for challenges to biologic patents was 38%. Then in FY18, when the overall institution rate dipped 3% to 60%, the institution rates for petitions challenging Orange Book-listed patents dropped 30% to 47%, and the biologic petition institution rate went up 11% to 49%.
Fig. 3. Trend in Institution Rates[3]
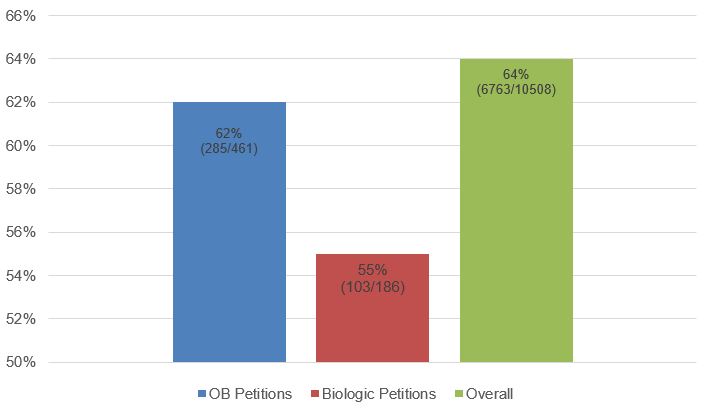
As shown in Fig. 4 below, the institution rate for petitions challenging Orange Book-listed and biologic patents was lower than the institution rate for petitions overall. In addition, the institution rate for challenges to biologic patents was even lower than for Orange Book-listed patents. Again, although the data sample for Orange Book-listed and biologic patents is much smaller than that for IPR petitions overall, these data may reflect a greater level of unpredictability (and, therefore, nonobviousness) for inventions in the small and large molecule space compared to other types of inventions, and for large molecule inventions compared to small molecule inventions.
Fig. 4. Institution Rate for OB and Biologic Petitions Compared to Overall Institution Rate (Sept. 16, 2012-June 30, 2021) [4]
Nevertheless, even though petitions challenging Orange Book-listed patents have a greater chance of institution than petitions challenging biologic patents, once instituted, Orange Book-listed patents fare better, with more than twice the percentage of all challenged claims surviving (49% compared to 23%). Indeed, the 49% claim survival rate for petitions challenging Orange Book-listed patents is closer to the 53% overall claim survival rate than it is to the 23% biologic claim survival rate. While these differences could be a statistical artifact due to the smaller number of biologic cases, the data may reflect the wider diversity of challenged biologic patents compared to Orange Book-listed patents, which do not include patents directed to methods of making, packaging, or metabolites/intermediates.
Fig. 5. Final Written Decision Outcomes[5]
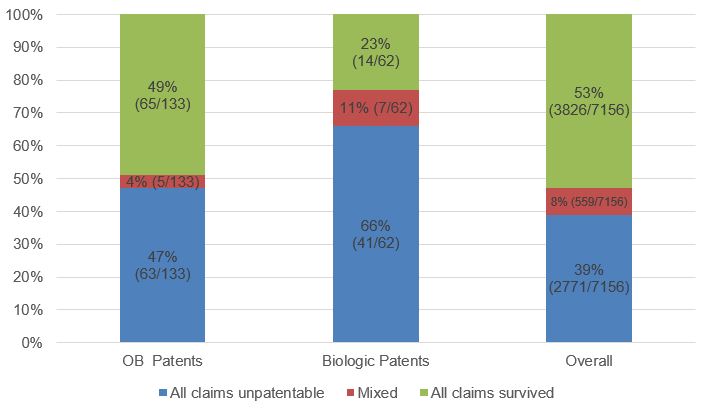
The USPTO report also presents Final Written Decision outcomes by number of challenged patents and by number of challenged claims. The data showed trends similar to the claim survival rates presented above based on the number of petitions. For Orange Book-listed patents, the Final Written Decision outcomes by patent were: 45% all claims unpatentable, 5% mixed outcome, and 50% all claims survived.[6]For biologic patents, the Final Written Decision outcomes by patent were: 70% all claims unpatentable, 6% mixed outcome, and 24% all claims survived.[7]For Orange Book-listed patents, the Final Written Decision outcomes by claim were: 42% unpatentable and 58% survived.[8]For biologic patents, the Final Written Decision outcomes by claim were: 72% unpatentable and 28% survived.[9]
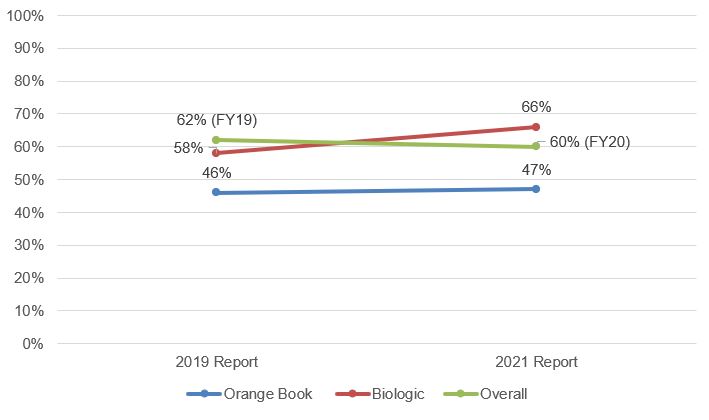
The 2019 and 2021 USPTO reports show that Final Written Decision outcomes have been worsening for patent owners of biologic patents and remained relatively steady for patent owners of Orange Book-listed patents. Again, while these differences could be a statistical artifact due to the smaller number of biologics cases, the data may reflect the wider diversity of challenged biologic patents compared to Orange Book-listed patents.
Fig. 6. Trend in Final Written Decisions Where All Claims Were Found Unpatentable[10]
Ultimately, each PTAB challenge is unique; however, the 2021 Report can serve as a tool for patent owners and petitioners in developing strategies for challenging and defending Orange Book-listed and biologic patents.
FOOTNOTES
[1]Sources: FY21 Q3 (June 2021) Update PTAB Orange Book patent/biologic patent study; Orange Book patent/biologic patent study and district court pharma litigation study, July 2019; and New PTAB Studies in AIA Proceedings: Expanded Panels and Trial Outcomes for Orange Book-listed Patents, March 2018. March 2018 Study time period is Sept. 16, 2012 - Sept. 30, 2017. July 2019 Study time period is Sept. 16 2012 - Nov. 30, 2018. August 2021 Study time period is Sept. 16, 2012 - June 30, 2021.
[2]Source: FY21 Q3 (June 2021) Update PTAB Orange Book patent/biologic patent study, Figures 2 and 3.
[3]Source: FY21 Q3 (June 2021) Update PTAB Orange Book patent/biologic patent study Fig. 7 and Fig. 5.
[4] Source: FY21 Q3 (June 2021) Update PTAB Orange Book patent/biologic patent study, Fig. 7.
[5]Source: FY21 Q3 (June 2021) Update PTAB Orange Book patent/biologic patent study Figs. 10 and 11 and DocketNavigator 2020 Year in Review (2013-2020). Percentages reflect only outcomes with a substantive decision on the merits; the denominator is the total of all claims unpatentable + mixed + all claims survived. A “mixed” outcome is when at least one challenged claim is found unpatentable and at least one challenged claim survived unchanged.
[6]Source: FY21 Q3 (June 2021) Update PTAB Orange Book patent/biologic patent study, Fig. 12. Percentages reflect only outcomes with a substantive decision on the merits; the denominator is the total of all claims unpatentable + mixed + all claims survived. A “mixed” outcome is when at least one challenged claim is found unpatentable and at least one challenged claim survived unchanged.
[7]Source: FY21 Q3 (June 2021) Update PTAB Orange Book patent/biologic patent study, Fig. 13. Percentages reflect only outcomes with a substantive decision on the merits; the denominator is the total of all claims unpatentable + mixed + all claims survived. A “mixed” outcome is when at least one challenged claim is found unpatentable and at least one challenged claim survived unchanged.
[8]Source: FY21 Q3 (June 2021) Update PTAB Orange Book patent/biologic patent study, Fig. 14.
[9]Source: FY21 Q3 (June 2021) Update PTAB Orange Book patent/biologic patent study, Fig. 15.
[10] Source: FY21 Q3 (June 2021) Update PTAB Orange Book patent/biologic patent study Figs. 10 and 11; Orange Book patent/biologic patent study and district court pharma litigation study, July 2019, Fig. 6;




 />i
/>i

