As of July 13, 2021, four generic declarations have been filed so far on China’s new patent linkage registration system. In compliance with the Phase One Trade Agreement, China implemented a patent linkage system in their amended patent law effective June 1, 2021. The Chinese patent linkage system prevents marketing approval of generic drugs until after the expiration of patents covering the drugs or uses. The 4 declarations are all from Chinese companies for drugs from Western companies including GlaxoSmithKline (GSK), Novartis and Pfizer.

Generics can make the following declarations:
-
There are no relevant patents registered on the platform;
-
The relevant patent has lapsed or was declared invalid or the generic applicant has a license;
-
This is a registered patent but the generic applicant will not sell the drug before expiration of the patent.
-
There is a registered patent on the platform, but it will be declared invalid or the generic is outside the scope of protection of the patent.
The 4 declarations are all from Chinese companies for drugs from Western companies including GlaxoSmithKline (GSK), Novartis and Pfizer. However, only the certification for a Pfizer generic is based on a registered drug (class III) while the other certifications are class I indicating no drug has been registered on the platform (not that there is no relevant drug). These Western companies now have 45 days from publication to either file an administrative adjudication with the China National Intellectual Property Administration or litigation with the Beijing Intellectual Property Court although GSK and Novartis will need to register their patents first.
Within 15 days of receiving a Notice of Acceptance from the Court or CNIPA, the holder must notify the Center for Drug Evaluation (CDE) with a copy of the same or a Notice of Docket and notify the generic drug applicant. The CDE will then impose a 9-month moratorium on marketing authorization approval but will not stop the evaluation of the generic’s application. Note that generics of biologics and TCMs are excluded from the 9-month moratorium. Accordingly, if no decision is reached within 9 months, the generic’s application could be approved shortly thereafter making the speed of the dispute resolution essential.
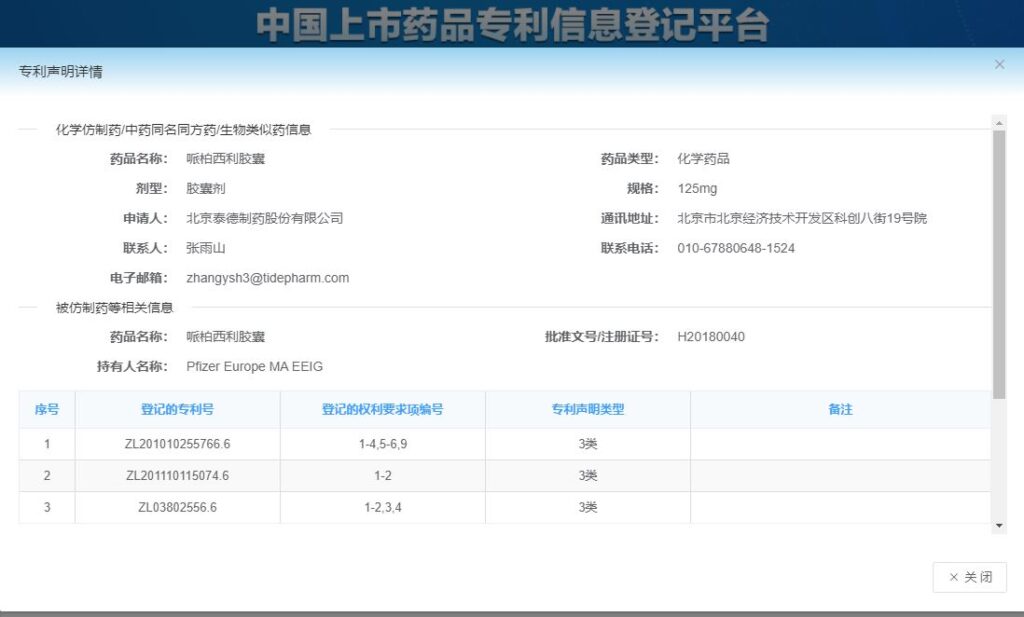
This Class 3 declaration lists Pfizer’s patents and relevant claims.



 />i
/>i

