As reported in our August 7, 2024, blog item, the U.S. Food and Drug Administration (FDA) announced on August 1, 2024, that it will hold a public meeting on September 25, 2024, on the development of an enhanced systematic process for its post-market assessment of chemicals in food. 89 Fed. Reg. 65633. FDA has posted a Discussion Paper Development of an Enhanced Systematic Process for the FDA’s Post-Market Assessment of Chemicals in Food (Discussion Paper). The Discussion Paper “broadly outlines a general approach for such a systematic process that would allow the FDA to proactively identify and target chemicals currently in the food supply for assessment in a structured manner based on risk.” The purpose of the Discussion Paper is to obtain public comment on the process to assist FDA in developing the post-market chemicals program that it will establish under the new FDA Human Foods Program. According to the Discussion Paper, FDA will begin the post-market assessment process with a review of information before conducting either a Focused or Comprehensive Assessment:
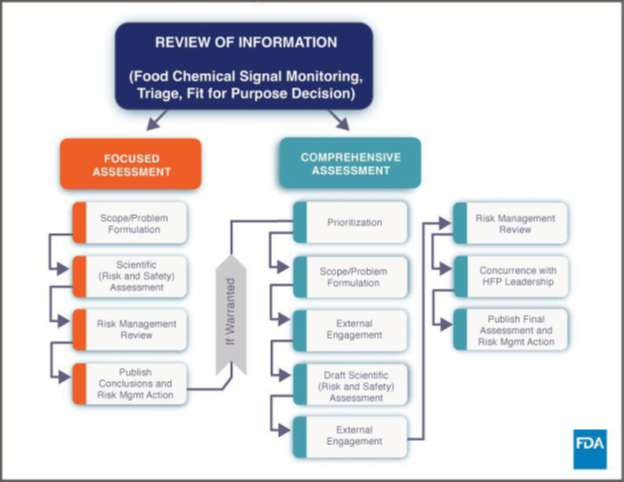
FDA will use answers to the following Fit for Purpose Decision Tree questions to help determine if an assessment should be Focused or Comprehensive:
- Will the assessment require significant resources outside of the Office of Post-market Assessment that require prioritization of risk within the new FDA Human Foods Program?
- Is there scientific consensus and/or strong weight of evidence about the substance suggesting its potential to impact the prevailing conclusion of reasonable certainty of no harm under the conditions of use in food?
- Have multilateral organizations, U.S.-bilateral organizations, and/or scientific organizations recently reviewed the risks associated with the food substance and identified potential safety concerns?
- Is there evidence of a change in dietary exposure indicative of an impact to consumer health?
- Is the substance of significant public health interest?
- Are there statutory deadlines or other required timelines for FDA to make its determination?
The Discussion Paper states that FDA “seeks to develop an objective post-market assessment prioritization of risk process that is sufficiently flexible while ensuring the process is science-based, data-driven, systematic, and reproducible.” FDA envisions using a Multi-Criteria Decision Analysis (MCDA) method, wherein the higher the total score, the higher the priority for that chemical for further review, with the primary focus being risk to public health (risk ranking). For public health ranking, FDA “tentatively envision[s] that a chemical that would receive a higher public health score is one for which:”
- The toxicity of the chemical is severe with potentially life-threatening adverse health effects;
- Changes in exposure have occurred (for example, contamination data indicate significantly higher levels than previously documented, consumption of the foods in which the chemical is found has increased, and/or there has been a significant increase in production volume of the chemical compared to the previous assessment);
- The chemical is found in or could be present in food intended for vulnerable subpopulations; and
- Newly available information, data, or science indicates a potentially significant impact on the conclusions of the previous assessment of the chemical.
The Discussion Paper notes that additional criteria that may be considered include, for example, interest and/or attention to this chemical by other organizations or the public.
The Discussion Paper includes the following questions for public comment:
- When and how should FDA engage the public on post-market assessments?
- Are the frequency and mechanisms of the envisioned public engagement appropriate? If not, please provide alternative areas for engagement/communication, additional information that should be shared publicly, and the rationale for the change.
- Should FDA integrate an advisory committee review into its post-market assessment process? If yes, at what stage, and what should the committee’s role be?
- Are the Fit for Purpose Decision Tree questions appropriate? If not, what questions should be added or modified to be more appropriate to the task?
- Is the Prioritization of Risks scheme appropriate for ranking food chemicals (including contaminants, food ingredients, and those substances used in contact with food) for post-market assessments? If not, please explain why and how the Prioritization of Risks scheme should be modified. Please provide supporting rationale for the changes.
- Is FDA’s two-pronged approach of Focused Assessments and Comprehensive Assessments appropriate to assess public health risks of chemicals in food? If not, please explain why and provide an alternative process, including rationale for such alternative(s).
Comments regarding the questions in the Discussion Paper should be submitted to Regulations.gov Docket No. FDA-2024-N-3609 by December 6, 2024.




 />i
/>i

