On Thursday, January 13, 2022, the Supreme Court of the United States issued its opinions in each of the Centers for Medicare and Medicaid Services (CMS) and Occupational Safety and Health Administration (OSHA) vaccination requirement cases in Biden v. Missouri and Nat’l Federation of Independent Business v. Dept. of Labor, permitting the federal vaccine mandate for nearly 10 million healthcare workers to go forward pending further review, but preventing OSHA’s enforcement of its vaccine or test mandate for workers of employers with 100 or more employees.
In Biden v. Missouri, the Court stayed the lower courts’ preliminary injunctions against CMS’s enforcement of its Interim Final Rule, implemented in November 2021. The rule created a new Condition of Participation (CoP) for certain Medicare and Medicaid-certified facilities, requiring that in order to continue to receive Medicare and Medicaid reimbursements under the new rule, facilities must ensure that 100% of their staff – unless exempt for medical or religious reasons – are vaccinated against COVID-19. The Supreme Court’s ruling removes the barrier of enforcement of the CMS vaccine mandate imposed by the preliminary injunctions against enforcement effected for 24 states (other than Texas), granted by the federal district courts for the Eastern District of Missouri and Western District of Louisiana, respectively, issued in late 2021, pending a final ruling in each of the lower court cases on appeal to the appellate courts for the Fifth and Eighth circuits. A separate case controlling the fate of the CMS vaccine mandate’s enforceability in Texas is still pending, and thus the mandate remains blocked in the State of Texas. CMS has, however, requested Judge Matthew Kacsmaryk of the U.S. District Court for the Northern District of Texas to similarly lift its preliminary injunction against enforcement of the mandate in Texas in light of the Biden v. Missouri decision. Thus, for now, the CMS vaccine mandate for healthcare employees will be allowed to proceed in adjusted scheduled phases, with all employees of facilities in the 24 states bound by the Supreme Court’s decision required to obtain their first COVID-19 vaccine dose by February 13, 2022. Employees of facilities in the other 25 states (except Texas) that did not challenge the CMS vaccine mandate must obtain their first COVID-19 vaccine dose by January 27, 2022, according to CMS’s originally scheduled phases under the Interim Final Rule.
Conversely, in Nat’l Federation of Independent Business v. Dept. of Labor, the Court stayed OSHA’s enforcement of its emergency temporary standards requiring employees of firms with greater than 100 employees to be vaccinated against COVID-19, meaning the OSHA vaccine mandate cannot be implemented.
The majority of the Court ultimately held in its per curiam opinion in Biden v. Missouri that the CMS vaccine mandate is within the powers granted by Congress to the Secretary of Health and Human Services to require that the healthcare facilities covered by CMS’s Interim Final Rule ensure that their employees be vaccinated against COVID-19 to continue to receive federal reimbursement.
The CMS Vaccine Mandate
The CMS staff vaccination requirement applies to almost all healthcare facilities that receive Medicare and Medicaid reimbursements – accordingly, skilled nursing facilities and facilities that care for individuals with disabilities will be required to implement the mandate. Assisted living facilities are not included in the CMS Interim Final Rule and are also not subject to any current federal COVID-19 vaccine requirement in light of the decision regarding the OSHA vaccine or testing requirement. However, CMS notes in a recent External FAQ Guidance Memo that long-term care staff who move between facilities (e.g., assisted living facilities and nursing homes) and provide care, treatment or other services for the nursing home or its residents – whether under contract or other arrangement – must be vaccinated in accordance with the Interim Final Rule.
The CMS vaccine mandate applies to all eligible “staff” working at CMS-certified facilities that participate in Medicare and Medicaid programs, regardless of clinical responsibility or patient contact. “Staff” includes facility employees, licensed practitioners, students, trainees, and volunteers, as well as individuals who provide care, treatment, or other services for the facility or its patients under contract or other arrangements. Therefore, many vendors of covered facilities will find that facilities are requesting that their employees be vaccinated as well. Notably, the mandate is not limited to staff who perform their duties solely on-site within a formal clinical setting – for example, staff providing off-site care such as home health – and as such all staff who interact with patients, residents, and other staff beyond the formal clinical setting (such as homes, clinics, administrative offices, off-site meetings, etc.) must be vaccinated. However, the CMS vaccine mandate does not apply to employees, staff or contractors who provide services 100% remotely and who do not have any direct contact with patients and other staff.
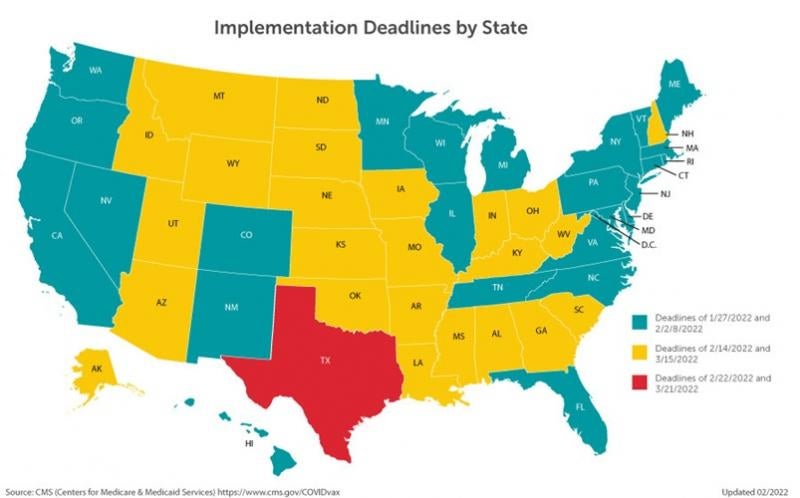
CMS’s implementation of its vaccine mandate is scheduled to go into effect in phases, with Phase 1 beginning January 27, 2022 for the 25 states and the District of Columbia (the “Early Compliance States")1 that did not challenge the mandate, and February 13, 2022, for the 24 states (the "Late Compliance States")2 bound by the recent Supreme Court ruling. Phase 2 begins February 28, 2022, for the Early Compliance States, and March 15, 2022 for the Late Compliance States.
Phase 1
By the applicable Phase 1 deadline, 100% of facility staff for facilities subject to the CMS vaccine mandate must have received their first dose of an approved COVID-19 vaccine, except those with or pending an exemption request or those having a temporary delay recommended by the CDC. Facilities subject to the CMS vaccine mandate must also have developed and implemented policies and procedures recommended by CMS in accordance with the Interim Final Rule by the applicable Phase 1 deadline.
Phase 2
By the applicable Phase 2 deadline, 100% of facility staff at facilities subject to the CMS vaccine mandate must have received their first dose of an approved COVID-19 vaccine, except those with a granted exemption request and those having a temporary delay recommended by the CDC. "Fully vaccinated" means the person has received a single-dose vaccine, or all required doses of a multi-dose vaccine (e.g. two doses of Pfizer or Moderna vaccine). Booster doses are not included in the vaccination requirement.
Texas
Texas filed its own lawsuit that was dismissed on January 21, 2022, resulting in it having its own deadlines. Texas's Phase 1 deadline is February 22, 2022, and the Phase 2 deadline is March 21, 2022.
Enforcement
CMS will work directly with state survey agencies to regularly review compliance with the Interim Final Rule and expects the state survey agencies to conduct on-site compliance reviews during their standard recertification surveys and complaint surveys. Facilities with less than 100% staff vaccination compliance rates may be cited, but will be exempted from CMS enforcement actions (for example, civil monetary penalties or “CMPs”) if they meet the following criteria:
-
If by the applicable Phase 1 deadline, the facility has more than 80% staff vaccination compliance rate (i.e. at least first dose) and a plan to achieve a 100% staff vaccination compliance rate within 60 days; and
-
If by the applicable Phase 2 deadline, the facility has more than 90% staff vaccination compliance rate and a plan to achieve a 100% of staff fully vaccinated within 30 days.
Nursing homes found in violation of the CMS vaccine mandate may be subject to civil monetary penalties, denial of payment, and even termination from the Medicare and Medicaid program as a final measure.
Facilities failing to comply with the mandate (i.e., having a staff vaccination rate below 100% and/or failing to adequately implement recommended policies and procedures) will receive a citation with scope and severity to be based on the actual staff vaccination rate, number of COVID-19 cases at the facility, policy and procedure implementation, and infection control practices.
What This Means for Long-Term Care Facilities and Their Stakeholders
Long-term care facilities must act quickly – if they haven’t already – to take inventory of their staff vaccination rates and tailor their staffing strategy to comply with the CMS mandate. With the likelihood of some staff turnover resulting from this decision limiting facility census and ultimately impacting short-term cash flows, equity principals and lenders alike should expect turbulence in returns in the coming months, and must plan to work together accordingly to resolve any capital shortfalls or covenant defaults. No uncertain time is without opportunity, however, and expect dealmakers to come to the table with short-term liquidity financing solutions. At any rate, current stakeholders and potential investors in the long-term care industry would be wise to add vaccine mandate compliance to their due diligence questionnaires, as well as representations, warranties, and covenants in definitive agreements.
FOOTNOTES
[1] The 25 states that did not challenge the CMS vaccine mandate are: California, Colorado, Connecticut, Delaware, Florida, Hawaii, Illinois, Maine, Maryland, Massachusetts, Michigan, Minnesota, Nevada, New Jersey, New Mexico, New York, North Carolina, Oregon, Pennsylvania, Rhode Island, Tennessee, Vermont, Virginia, Washington and Wisconsin.
[2] The 24 states in which the previous preliminary injunctions against enforcement of the CMS vaccine mandate were struck down by the Biden v. Missouri decision are: Alabama, Alaska, Arizona, Arkansas, Georgia, Idaho, Indiana, Iowa, Kansas, Kentucky, Louisiana, Mississippi, Missouri, Montana, Nebraska, New Hampshire, North Dakota, Ohio, Oklahoma, South Carolina, South Dakota, Utah, West Virginia and Wyoming.



 />i
/>i
