Holding
In Mylan Labs. Ltd. v. Aventis Pharma S.A., IPR2016-00712, Paper 112 (P.T.A.B. Oct. 22, 2019), aff’d without opinion (Fed. Cir. Jan. 15, 2021), the Patent Trial and Appeal Board (“PTAB”) granted Patent Owner’s motion to amend, finding that Petitioner had not shown the proposed substitute claims to be unpatentable.
Background
The drug at issue was JEVTANA® (cabazitaxel), which is indicated for use in combination with prednisone for the treatment of patients with metastatic castration-resistant prostate cancer. Mylan challenged claims 1-5 and 7-30 of Orange Book-listed U.S. Pat. No. 8,927,592 (“the ’592 patent”) as unpatentable under 35 U.S.C. § 103(a). The ’592 patent, along with two continuation patents that issued from it, moved the patent expiration date for JEVTANA® out about five years beyond the other Orange Book-listed patents. This can be seen from the present Orange Book report on cabazitaxel:
|
Product 100 |
|||||||
|
Patent Data |
|||||||
|
Product No. |
Patent No. |
Patent Expiration |
Drug Substance |
Drug Product |
Patent Use Code |
Delist Requested |
Submission Date |
|
001 |
5847170 |
03/26/2021 |
DS |
DP |
|
|
|
|
001 |
5847170*PED |
09/6/2021 |
|
|
|
|
|
|
001 |
7241907 |
12/10/2025 |
DS |
|
|
|
|
|
001 |
7241907*PED |
06/10/2026 |
|
|
|
|
|
|
001 |
8927592 |
10/27/2030 |
|
|
U-1630 |
|
01/12/2015 |
|
001 |
8927592*PED |
04/27/2031 |
|
|
|
|
|
|
001 |
10583110 |
10/27/2030 |
|
|
U=2753 |
|
03/17/2020 |
|
001 |
10716777 |
10/27/2030 |
|
|
U=2856 |
|
07/21/2020 |
In addition, the 2030 expiration date of the ’592 patent is about 10 years after all regulatory exclusivity has expired:
|
Exclusivity Data |
||
|
Product No |
Exclusivity Code |
Exclusivity Expiration |
|
001 |
M-201 |
05/17/2020 |
|
001 |
M-209 |
09/14/2020 |
|
001 |
M-201 *PED |
11/17/2020 |
The PTAB instituted Mylan’s IPR, and thereafter Patent Owner, Aventis, filed a Response and a Contingent Motion to Amend. IPR2016-00712, Paper 112 at *2. In its Final Written Decision in September 2017, the PTAB concluded that Petitioner had shown by a preponderance of the evidence that claims 1-5 and 7-30 were unpatentable. The PTAB also denied Patent Owner’s Contingent Motion to Amend, holding that Patent Owner had not shown by a preponderance of the evidence that proposed substitute claims 31–34 were patentable. Id. at *3.
Patent Owner appealed, and the Federal Circuit vacated PTAB’s denial of Aventis’ Motion to Amend and remanded back to the PTAB for further consideration. Sanofi Mature IP v. Mylan Labs. Ltd., 757 F. App’x 988, 989 (Fed. Cir. 2019). According to the Federal Circuit, remand was appropriate in light of the decision in Aqua Products, Inc. v. Matal, 872 F.3d 1290 (Fed. Cir. 2017) (en banc), because the PTAB improperly placed the burden of proof on Aventis to establish that its proposed claims were patentable. In addition, according to the Federal Circuit, the PTAB incorrectly held that the preamble of proposed claim 31 should not be treated as a limitation. That incorrect claim construction led the PTAB to reject Aventis’ arguments of non-obviousness.
Proposed Substitute Claims
Proposed Claims 31-34, showing the changes from original claim 27, are reproduced below:
31. A method of increasing [the] survival [of a patient] comprising administering to a patient in need thereof
(i) an antihistamine,
(ii) a corticoid,
(iii) an H2 antagonist, and
(iv) a dose of 20 to 25 mg/m2 of cabazitaxel, or a hydrate
or solvate thereof,
wherein said antihistamine, said corticoid, and said H2 antagonist are administered prior to said dose of 20 to 25 mg/m2 of cabazitaxel, or hydrate or solvate thereof, in combination with prednisone or prednisolone, wherein said patient has [with a] castration resistant or hormone refractory, metastatic prostate cancer that has progressed during or after treatment with docetaxel [, comprising administering a dose of 20 to 25 mg/m2 of cabazitaxel, or a hydrate or solvate thereof, to the patient in combination with prednisone or prednisolone].
32. (substantively identical to original claim 28) The method according to claim 31, where the cabazitaxel, or hydrate or solvate thereof, is administered at a dose of 25 mg/m2.
33. The method according to claim 31, comprising repeating the administration of said antihistamine, said corticoid, said H2 antagonist, and said cabazitaxel, or hydrate or solvate thereof, as a new cycle every 3 weeks.
34. (substantively identical to original claim 30) The method according to claim 31, where the cabazitaxel, or hydrate or solvate thereof, is administered at a dose of 20 mg/m2.
PTAB Decision on Remand
Non-Obviousness
On remand the PTAB only reconsidered Patent Owner’s Motion to Amend and treated the preamble language “increasing survival” of claim 31 as a limitation. The PTAB stated:
[T]o prove a reasonable expectation of success with respect to a claim that recites a limitation setting forth achieving a particular result as the intended purpose for which a recited method must be performed, what is required is not proof that the recited method would actually bring about the recited result, but rather proof that a person of ordinary skill in the art would have had a reasonable expectation that performing the recited method would bring about the recited result.
IPR2016-00712, Paper 112, at *14.
Applying that standard, the PTAB concluded that Petitioner (bearing the burden of persuasion post-Aqua Products) did not establish that the proposed substitute claims would have been obvious. The preponderance of evidence did not support Petitioner’s position that a POSITA would have reasonably expected that administering cabazitaxel would increase survival of the claimed castration resistant or hormone refractory, metastatic prostate cancer patients that had progressed during or after treatment with docetaxel. Id. at *15. At best, a POSITA would have expected anti-cancer activity and hoped for, but not expected, increased survival. Id. at *16.
In reaching this conclusion, the PTAB highlighted testimony from Patent Owner’s expert, Dr. Sartor, that the results of the relevant clinical trial “surprised, delighted, [and] shocked him.” Id. at *18. The PTAB further noted that Petitioner’s expert, Dr. Seth, also provided evidence of no reasonable expectation of success. In particular, in response to a question about whether a person of ordinary skill in the art “would expect” an increase in survival, Dr. Seth responded that the person of ordinary skill in the art merely “would hope” to see such a result. Id. at *19.
The PTAB highlighted that there was significant evidence of record to establish that those skilled in the art knew that the indications of disease control do not always correlate with increased survival. And the PTAB buttressed that conclusion by examining clinical results from the prior art:
As for the overall survival shown in Pivot, the evidence of record does not support a finding that a median overall survival of docetaxel-resistant metastatic breast cancer patients of 12.3 months would have been interpreted as an increase in survival. First, Pivot did not report the overall survival for a control group or any other group whose results could be compared with the 12.3-month result. Ex. 1010, 1547–52. Accordingly, there is no way to determine from Pivot itself whether the overall survival reported in Pivot represented an increase, a decrease, or no significant change in overall survival.
Id. at *21.
The PTAB also gave weight to evidence of skepticism regarding the relevant clinical trial. Petitioner’s expert, Dr. Sartor, testified that: “it was a challenge to get the [TROPIC] study off the ground” because “some thought there was insufficient data with cabazitaxel in prostate cancer to safely move forward with a phase III study.” Furthermore, Dr. Sartor proffered that when the results of the relevant clinical trial were announced, “[p]ractitioners who had been aware of the existence of the study were surprised by the results.” Id. at *22.
After weighing the evidence, the PTAB found no reasonable expectation of success of arriving at the invention recited in the proposed substitute claims. Hence, the claims would not have been obvious.
Lack of Anticipation Based on Public Use
Petitioner also asserted that proposed substitute claims were anticipated by a public use of the claimed methods in the relevant clinical trials. Id. at *22. Patent Owner countered by urging that the proposed claims were not subject to the public use bar at the time of the clinical trials because “there is no evidence indicating that anyone involved with TROPIC (including the inventor) had determined that the claimed methods would work for their intended purpose.” Id. at *22-23.
Petitioner’s anticipation argument was rejected by the Board. The PTAB noted that the alleged public use was a clinical study and that the evidence of record did not support a finding that the subject matter of the proposed substitute claims was ready for patenting at the time of the study. “The existence of a phase III study to determine whether the claimed treatment method would achieve the intended purpose is strong evidence that the inventor had not yet determined that the invention would work for its intended purpose.” Id. at *24. Moreover, the Board noted that there was no disclosure of any of the premedication steps in the proposed claims. Id. at *25.
The Claimed Subject Matter Was Patent-Eligible
This case is also noteworthy because Petitioner raised the specter of lack of subject matter eligibility under 35 U.S.C. §101. Id. at *25. However, the PTAB rejected Petitioner’s §101 arguments. Under Step 2A of the Mayo/Alice two-step subject matter eligibility test, the Board noted that the proposed substitute claims recite an abstract idea (a mental process). Specifically, the PTAB found that “a method of increasing survival” recites an intentional purpose performed mentally and was an abstract idea. Id. at *28. But the PTAB found that under Vanda Pharms. Inc. v. West-Ward Pharms., 887 F.3d 1117, 1134 (Fed. Cir. 2018), “[w]hen a claim integrates a mental process step into a method of treatment, that claim is not directed to an abstract idea.” Id. at *29. In other words, even having found that the claims recite a mental process, the PTAB concluded the process was incorporated into a practical application that was eligible for patent protection.
Take-Aways
Although the rate of granting proposed substitute claims is higher now than it used to be (15% on a per claim basis as of Nov. 30, 2020, compared to 4% in December 2017), it is still rare for a Patent Owner to have a Motion to Amend granted. And that is true, even considering the post-Aqua Products shift of the burden to the Petitioner to show unpatentability rather than resting on the Patent Owner to show patentability.
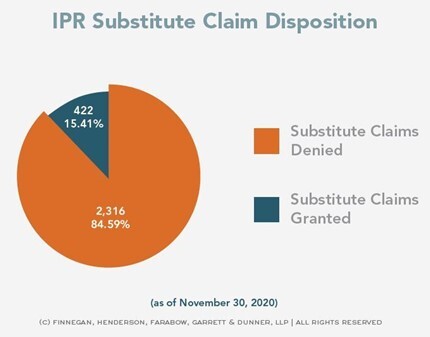
Source: https://www.finnegan.com/en/america-invents-act/claim-and-case-disposition.html
In fact, it actually is not very common for Patent Owners even to file a Motion to Amend. According to the Patent Trial and Appeal Board Motion to Amend Study, of 5359 trials between Oct. 1, 2012 and March 31, 2020, 80% were completed without a Motion to Amend and another 10% are pending without a Motion to Amend. Source: https://www.uspto.gov/patents/ptab/motions-amend-study). That leaves only 10% of trials with a Motion to Amend. The same report shows the raw number of Motions to Amend is only 568 from trials filed from Oct. 1, 2012 through Mar. 31, 2020. The PTAB’s Motion to Amend Pilot Program may encourage more Patent Owners to file, but probably a few more decisions granting proposed substitute claims will be stronger encouragement.
Moving beyond these statistics, this PTAB decision provides an example of different outcomes depending on whether the claim preamble is construed as a limitation. Also, when clinical trials are applied as prior art, the PTAB gives useful guidance as to how to defeat public use based on the existence of the trials at a relevant time. This is a key consideration for innovative pharmaceutical companies. And finally, amidst the great uncertainty of subject matter eligibility under 35 USC §101, the decision provides an example of successfully arguing subject matter eligibility, even if the claims recite a mental process, which is a type of abstract idea, as long as the process is incorporated into a practical application a la Vanda.




 />i
/>i

