The Centers for Medicare & Medicaid Services (CMS) released a Final Notice for Transitional Coverage for Emerging Technologies (TCET), effective August 12, 2024, to provide “transparent, predictable, and expedited national coverage” for eligible devices designated as “breakthrough devices” by the U.S. Food & Drug Administration (FDA). FDA-designated breakthrough devices are devices that FDA has determined provide for more effective treatment or diagnosis of life-threatening or irreversibly debilitating human disease or conditions. As CMS noted in the background section to the Final Notice, the TCET pathway is intended to provide a clear, transparent, and consistent coverage process for these devices.
The Goal of the TCET Pathway is to Expand Access to Critical Technology
Interestingly, just because FDA has approved or cleared a medical device does not mean that CMS will cover (and reimburse) the device for Medicare beneficiaries. The Final Notice states that the time between when a device is approved or cleared by FDA and when a device obtains a national coverage determination (NCD) generally takes nine to twelve months. For certain FDA-designated breakthrough devices, this gap can significantly delay access to critical and innovative technology for Medicare beneficiaries. Because some commercial payers follow Medicare coverage guidelines, the delay may impact more than just Medicare beneficiaries.
The much-anticipated Final Notice implements a new voluntary TCET pathway that provides manufacturers of FDA-designated breakthrough devices an opportunity to expedite the Medicare coverage process and reduce uncertainty about coverage through a pre-market evaluation of potential harms and benefits of devices. This approach will allow product manufacturers to identify evidence gaps and address those gaps early in the process to obtain Medicare coverage. The TCET pathway is intended to provide Medicare patients with more timely and predictable access to these new, innovative technologies.
CMS made several changes between the proposed notice and the Final Notice, such as (1) providing an opportunity for manufacturers to submit a non-binding letter of intent; (2) revising the nomination review process; (3) revising interim reporting requirements; and (4) clarifying anticipated CMS communication throughout the process. CMS has also expressed intent to release certain proposed factors that CMS will use to prioritize TCET nomination.
TCET Program Eligibility
Interested manufacturers must submit a self-nomination packet. Due to CMS resource constraints, the agency anticipates accepting approximately five devices per year as TCET candidates.
The Final Notice finalizes the four criteria for program eligibility as previously proposed. The pathway will be available to devices that:
- have breakthrough designation from FDA,
- are determined to be within a Medicare benefit category,
- are not already subject to an existing Medicare NCD, and
- are not otherwise excluded from coverage through statute or regulation.
Whether diagnostic laboratory tests are eligible for the program will be determined on a case-by-case basis. In addition, if a device has already received FDA market authorization (or such authorization is anticipated within six months), it is not eligible for the program.
Evidence Preview
CMS typically begins review of whether a device is eligible for coverage after FDA completes its market authorization process. In order for Medicare to cover an item or service, the item or service: (1) must fall within at least one Medicare benefit category established by statute, (2) must not be specifically excluded by statute, and (3) must be “reasonable and necessary” for the diagnosis or treatment of a disease or impairment as determined through an evidence-based process.
To facilitate this evidence-based process, the TCET pathway finalizes CMS’s ability to conduct an “Evidence Preview”, where CMS will review evidence provided with a TCET nomination package, via third-party contractors, to provide early feedback on the strength and weaknesses of the evidence before FDA decides on marketing authorization. In addition to the proposed process, the Final Notice adds additional policies that allow CMS to preemptively conduct a clinical endpoint review and potentially include a Medicare Coverage Advisory Committee (MedCAC) assessment to align on evidence gaps in the Evidence Preview stage. Once the Evidence Preview is finalized, the manufacturer may choose to pursue national coverage by submitting a formal NCD request — or withdraw from the TCET program. If a manufacturer withdraws at this point, CMS will still publish the Evidence Preview. The Final Notice, however, clarifies that CMS will provide a summary of the evidence rather than sharing the full Evidence Preview with the Medicare Administrative Contractors (MACs) as initially proposed. If CMS finds evidence gaps, manufacturers may propose an “Evidence Development Plan (EDP)” to address the gaps. CMS will meet with the manufacturer to discuss the EDP and the manufacturer will have 60 business days to revise its submission before finalizing.
Temporary Coverage
The proposed notice stated that temporary coverage would be provided for three to five years; however, the Final Notice declines to provide a time frame and instead suggests that coverage will be tied to the EDP and whether the manufacturer sufficiently addresses any identified evidence gaps.
The proposed notice also stated that CMS intends to finalize a TCET NCD for TCET program participants within six months after FDA market authorization; however, the Final Notice notes that this timeframe may be impossible depending on the proposed EDP.
CMS also finalized a policy to apply coverage under a TCET NCD to “similar devices.” CMS does not define “similar devices”; however, the devices must be breakthrough devices and are subject to the same coverage conditions (including a requirement to propose an EDP). CMS also declined to establish a “first-to-market” exclusivity period.
Timeline
In the Final Notice, CMS changed the timeframe for reviewing TCET nominations. The Final Notice states that CMS will review nominations on a quarterly basis, as opposed to within 30 days of submission as previously proposed. In addition, CMS will permit manufacturers to submit a non-binding letter of intent to nominate a potentially eligible device between 18-24 months before FDA authorization is expected and will continue to consider nominations approximately twelve months before the anticipated date of market authorization. The TCET process, however, will begin approximately one year before the anticipated FDA marketing authorization.
CMS provides the below timeline summarizing the TCET process:
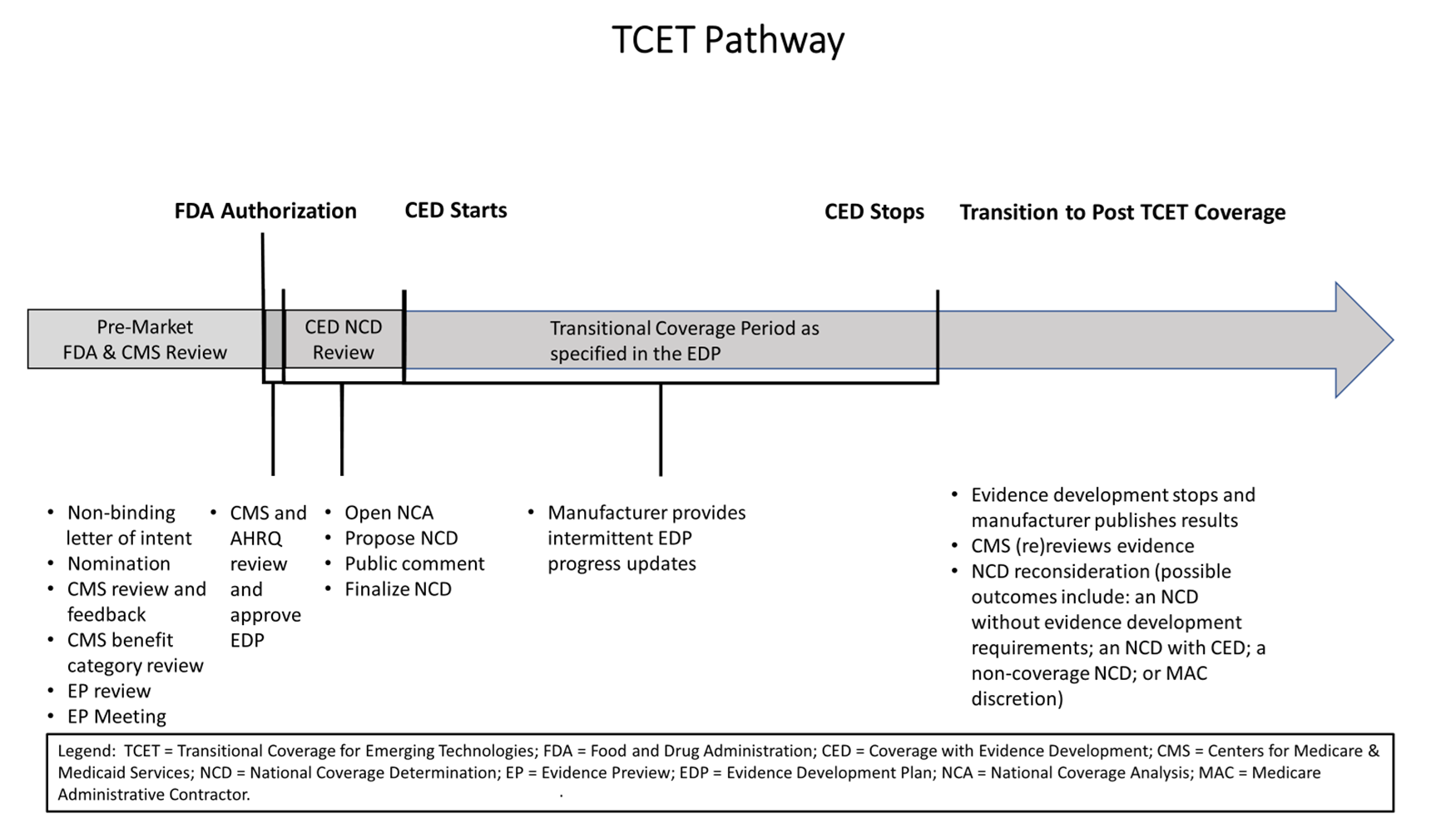
Source: 89 Fed. Reg. 65724, 65753 (Aug. 12, 2024).
Next Steps for Manufacturers Interested in the TCET Pathway
As noted above, CMS will accept only a very small number of applicants into the TCET pathway. Manufacturers will need to assess their strategic options as to whether the TCET pathway is one they wish to pursue. If so, manufacturers should begin at an early stage in their product development to assure that they have identified an applicable Medicare benefit category allowing coverage, and that they are assembling evidence sufficient to convince CMS to accept them into the pathway.
Manufacturers interested in the TCET pathway can notify CMS of their interest electronically via the Coverage Center Web site using the “Contact Us” link available here. To be considered for the first quarterly review, nominations will need to be submitted by October 31, 2024. The deadlines for the next three quarterly review cycles are January 31, 2025, April 30, 2025, and July 31, 2025.






 />i
/>i

