On June 12, 2014, the US Court of Appeals for the Federal Circuit issued a precedential opinion affirming the obviousness of a patent claim directed to a drug molecule. Bristol-Myers Squibb Co. v. Teva Pharms. USA, Inc., ___ F.3d ___ (2014). This is an example of the Federal Circuit holding a molecule patent invalid for obviousness.
The Federal Circuit upheld US District Court for the District of Delaware Magistrate Judge Christopher Burke's opinion that held claim 8 of U.S. Patent No. 5,206,244 invalid in light of a structurally similar molecule. Claim 8 covers the entecavir molecule, which is the active ingredient in BMS' Baraclude® tablets, which are designed to treat hepatitis B virus (HBV) infection. Teva successfully argued that one of ordinary skill in the art seeking to make an anti-HBV drug in October 1990 would have selected a prior art compound called 2'-CDG as a "lead compound" and would have modified it by adding a methylene (i.e. carbon-carbon double bond) group as indicated in the diagram to the right, below.
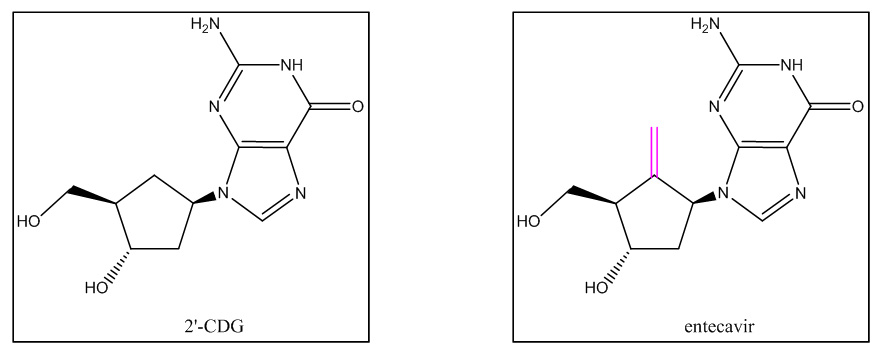
Lead Compound
Relying on testimony from both parties' experts and prior art publications, the Federal Circuit saw no error in the district court's finding that 2'-CDG was a proper lead compound. There was sufficient evidence that one of ordinary skill would have studied carbocyclic nucleosides generally, and 2'-CDG specifically, as antiviral drugs, especially because BMS' expert admitted that "medicinal chemists . . . were actually treating and using 2'-CDG as a lead compound" in the search for new antivirals. Slip. Op. at 9-10. BMS argued that 2'-CDG would not be a lead compound because it was discovered to be toxic after the filing date of the patent. Id. at 10. The Federal Circuit (and the district court) rejected BMS' argument because in October 1990 (i.e. the date of the proper inquiry), "2'-CDG was not yet known to have high toxicity" and BMS' expert agreed that researchers thought 2'-CDG was a promising compound at that time. Id. at 10.
Motivation to Modify
The Federal Circuit also found that the record amply supported the district court's conclusion on motivation to modify 2'-CDG to make the patented compound, entecavir. Slip Op. at 11-14. For example, both parties' experts agreed that chemists were making changes on the carbocyclic ring in the prior art, and Teva's expert stated that such changes resulted in greater activity than changes elsewhere on the molecule. Id. at 12. Unrefuted expert testimony also explained that the modification would take place at the 2' or 5' position on the carbocyclic ring because small changes could easily be made only at these positions. Id. at 12. The experts also agreed that the skilled artisan would focus on the smallest elements for the substitution, and BMS' expert stated that he would "rule out everything but the carbon." Id.The Federal Circuit found no clear error in the district court's finding that the modification required was a minor one based on the testimony of both parties' experts and a prior art article teaching improved antiviral activity by addition of a methylene group to a carbocyclic nucleoside. Id. at 12-13.
Reasonable Expectation of Success
Based on the prior art and the structural similarity of 2'-CDG and entecavir, the Federal Circuit found no error in the district court's finding of "reasonable expectation of success." Id. at 14. In doing so, the Federal Circuit rejected a bright-line rule regarding reasonable expectation of success in new chemical entities proposed by BMS. Specifically, BMS had argued that the existence of unexpected properties forecloses a finding of a reasonable expectation of success. Id. at 14. Citing its en banc In re Dillon decision, the Federal Circuit held that "unexpected results do not per se defeat, or prevent, the finding that a modification to a lead compound will yield expected, beneficial properties." Id. at 15. Instead, the court found that unexpected results should be analyzed as secondary considerations of nonobviousness. Id.
Secondary Considerations
The Federal Circuit found no reversible errors in the district court's analysis of secondary considerations of nonobviousness.[1] The Federal Circuit was deferential to the lower court's factual findings regarding evidence of secondary considerations, including unexpected results. Id. at 18. The district court found that the results regarding entecavir's high potency and large therapeutic window were not entirely unexpected because it was known in the prior art that 2'-CDG was effective against HBV and had a good therapeutic window. Id. They were differences of degree, not kind. Id. While the district court credited entecavir's high barrier to resistance as an unexpected property, the three properties taken together were not sufficient to support nonobviousness. Id. at 17-18.
[1] The Federal Circuit found that the district court made two legal errors (albeit harmless ones) in assessing unexpected results because it: (1) compared entecavir to another HBV drug on the market and not the closest prior art, 2'-CDG, and (2) looked at what the inventor knew, not a person of ordinary skill in the art. Id. at 18-19. The Federal Circuit also found no error in the lower court's findings on commercial success and long-felt need. Id. at 19.





 />i
/>i

