In just the US, more than 100,000 men, women, and children are waiting for an organ transplant. According to the United Network of Organ Sharing, more than 6,000 people in the US die on an annual basis waiting for a matched organ. Xenotransplantation is an important development in medical science and has the potential to reduce the global organ shortage. The 2016 FDA Guidance for Industry: Source Animal, Product, Preclinical and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans defines xenotransplantation as any procedure that involves the transplantation, implantation, or infusion into a human recipient of either (a) live cells, tissues, or organs from a nonhuman animal source; or (b) human body fluids, cells, tissues, or organs that have had ex vivo contact with live nonhuman animal cells, tissues, or organs.
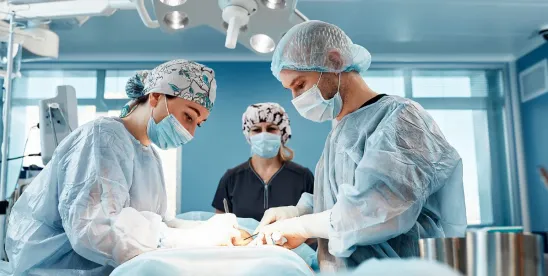
Recently, Massachusetts General Hospital announced the first transplant of a genetically engineered pig kidney into a 62-year-old male patient with end-stage kidney disease. The patient’s pig kidney was provided by eGenesis, a xenotransplantation therapy company in Cambridge, MA. eGenesis utilized CRISPR-Cas9 technology to modify 69 of the animal’s genes to improve the organ’s compatibility with humans and reduce the chances of rejection. The patient received the transplant on 3/21/24 and was discharged from the hospital on 4/4/24. He is now over a month out from the transplant, and the organ continues to function without rejection.
The pig kidney transplant was performed under a Food and Drug Administration protocol known as “expanded access” or “compassionate use” which provides access to drugs, medical devices, or biologics outside of clinical trials that have not yet been approved by the FDA. It is for patients who have a life-threatening illness and all other options have failed or are inaccessible to them. The policy dates back to the 1970s but was formalized through regulation in 1987 for drugs and biologics (aka, biological products), and in 1996 for devices. It was codified into law in 1997. [2018 Expanded Access Program Report]
The FDA Center for Biologics (CBER) regulates xenotransplantation products as biologics under authority of § 351 of the Public Health Service Act (42 U.S.C. 262) and the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 321 et seq.). According to FDA, a biologic is a product from natural sources, including human, animal, or other microorganisms, including living entities like blood, cells, tissues, viruses, toxins, antibodies, enzymes, proteins, and growth factors, among others. Per FDA, biologics are for the prevention, treatment, or cure of a disease or condition of human beings.
The legal framework surrounding xenotransplantation includes several key issues:
- Regulation and Oversight: Many countries have regulatory agencies, such as FDA, that oversee the safety and efficacy of medical treatments, including xenotransplantation. These agencies set standards for clinical trials, evaluate risks versus benefits, and monitor outcomes to ensure public health safety.
- Informed Consent: Among other things, informed consent should include informing the patient of the probability of success of the xenotransplant and the risks of rejection. Given zoonotic infectious risks to the recipient and recipient’s family or other intimate contacts, the FDA advises that the patient agree to inform contacts of their potential risks from the source animal species.
- Liability: Should informed consent be challenged, determining liability in cases where xenotransplantation leads to unforeseen adverse effects or transmittal of new diseases may be complex. As this nascent field emerges, we have not yet encountered circumstances in which legal action might be considered by families affected by unexpected consequences.
- Intellectual Property: Xenotransplantation involves significant research and development, often leading to patents and other forms of intellectual property protection. This raises legal issues such as the extent to which modifications made to an organism are patentable.
Xenotransplantation is an evolving area of medical science that may one day help the thousands of patients waiting for an organ transplant. We will continue to monitor the companies that are spearheading this exciting area of science and track other organs, including the heart, liver, and lungs, that are being studied.



 />i
/>i

