In Allergan USA, Inc. v. MSN Laboratories Private Ltd., No. 2024-1061 (Fed. Cir. August 13, 2024), the Federal Circuit reversed the District Court of Delaware’s invalidity determination of certain claims of U.S. Patent No. 7,741,356 (“the ’356 patent”) for obviousness-type double patenting. The Federal Circuit also reversed the district court’s invalidity determination of certain claims of U.S. Patents Nos. 11,007,179 (“the ’179 patent”), 11,090,291 (“the ’291 patent”), 11,160,792 (“the ’792 patent”), and 11,311,516 (“the ’516 patent”) for lack of written description.
Background
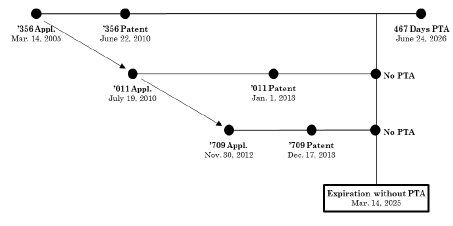
The ’356 patent issued from the first patent application to cover the pharmaceutical compound eluxadoline and accrued a patent term adjustment (“PTA”) of 1,107 days due to delay of prosecution. Applicant disclaimed all but 467 of the 1,107 days in order to benefit from patent term extension (“PTE”) stemming from delays in FDA approval. While prosecution for the ’356 patent’s application was pending, Applicant filed chains of continuation and divisional applications claiming benefit to the ’356 patent’s application. Of these, U.S. Patent No. 8,344,011 (“the ’011 patent”) issued on Jan. 1, 2013 and U.S. Patent No. 8,609,709 (“the ’709 patent”) issued on Dec. 17, 2013. Both patents expired Mar. 14, 2023, 20 years measured from the priority claimed to the ’356 patent application, because neither patent received PTA. The ’709 patent is subject to a terminal disclaimer over the ’356 patent. The Federal Circuit included a diagram on page 5 of its opinion, showing the timeline and relationship between these patents and their applications (excluding the PTE which is not relevant for the ODP analysis):
The ’179, ’291, ’792, and ’516 patents recite tablet formulations that are common in claiming 75 mg of eluxadoline.
Procedural History
One of the defendants in the case, Sun Pharmaceuticals submitted an Abbreviated New Drug Application (“ANDA”) seeking FDA approval to market and sell a generic version of Allergan’s eluxadoline tablet Viberzi. Viberzi had been approved in 2015 and marketed for treating symptoms of irritable bowel syndrome. Sun submitted a Paragraph IV certification pursuant to 21 U.S.C. § 355(j)(2)(A)(vii)(IV), contending that the claims of the ’356 patent (which covers the chemical compound eluxadoline) are invalid or would not be infringed by Sun’s generic product. Sun notified Allergan of the certification. In response, Allergan sued Sun in the District of Delaware under 35 U.S.C. § 271(e)(2)(A) for directly infringing claim 40 of the ’356 patent. While the ’356 case was pending, the ’179 patent (covering formulations using eluxadoline) issued and Allergan brought a second case against Sun asserting the ’179 patent. The ’291, ’792, and ’516 patents subsequently issued and were added to Allergan’s complaint in the new case.
Following a three-day bench trial, claim 40 of the ’356 patent that was asserted against Sun Pharmaceutical was found invalid under the doctrine of obviousness-type double patenting (“ODP”). Allergan argued claim 40 of the ’356 patent should not be subject to ODP over the claims of its children (’011 and ’709 patents) that were both filed later and issued later than the ’356 patent, because it was the first patent claiming eluxadoline to have been filed. The district court rejected this argument and considered only the expiration dates under In re Cellect, LLC, 81 F.4th 1216, 1228–29 (Fed. Cir. 2023).
Certain claims of the other patents (’179, ’291, ’792, and ’516 patents) asserted against Sun and other claims of these patents asserted against MSN were found invalid for lack of written description. The district court reasoned that the claim’s recital of an optional glidant as optional was not supported by the specification, because it did not specifically disclose a pharmaceutical tablet without a glidant (e.g., colloidal silica).
Allergan appealed the invalidity determinations of the claims asserted against Sun Pharmaceutical.
Issue(s)
Whether claim 40 of the ’356 patent is invalid for obviousness-type double patenting (“ODP”).
Whether the asserted claims of the ’179, ’291, ’792, and ’516 patents are invalid for lack of written description.
Holding(s)
Under the doctrine of ODP, a first-filed, first-issued, later-expiring claim cannot be invalidated by a later-filed, later-issued, earlier-expiring reference claim having a common priority date.
The district court clearly erred in finding that the specification does not reasonably convey to a person of ordinary skill in the art that the inventors had possession of a formulation without a glidant.
Reasoning
As to the ODP invalidation of the ’365 patent, the Federal Circuit explained the purpose of the ODP doctrine “is to prevent patentees from obtaining a second patent on a patentably indistinct invention to effectively extend the life of a first patent to that subject matter.” Allergan v. MSN Labs. at 16. The ’365 patent is the first-filed, first-issued patent in its family. Therefore, it “sets the maximum period of exclusivity for the claimed subject matter and any patentably indistinct variants.” Id. at 15. The Federal Circuit concluded that the later-filed, and later-issued claims of the ’011 and ’709 reference patents are not proper ODP references for invalidating the’356 patent’s claims. Id. at 16.
The Federal Circuit further noted that allowing a first-filed, first-issued parent patent having duly received PTA to be invalidated with a later-filed, later-issued child patent would amount to requiring patent owners to file a terminal disclaimer to disclaim only PTA. The Federal Circuit reasoned that this would abrogate the benefit of PTA guaranteed by 35 U.S.C. § 154(b). Id. at 20.
The Federal Circuit clarified that Cellect did not address the question of under “what circumstances can a claim properly serve as an ODP reference.” Allergan v. MSN Labs. at 15. Therefore, the Federal Circuit explained that the Cellect holding is only controlling in this case to the extent that it requires consideration of the challenged patent’s expiration date after accounting for PTA. Id. at 15. Thus, Cellect does not compel invalidation under these facts.
The Federal Circuit also clarified that the holding of Gilead Scis., Inc. v. Natco Pharma Ltd., 753 F.3d 1208 (Fed. Cir. 2014), where a later-issued but earlier-expiring patent was held as a qualifying ODP reference to invalidate an earlier-issued but later-expiring patent, did not account for the role of filing dates in the ODP analysis. Id. at 18-19.
As to the invalidity determination of the ’179, ’291, ’792, and ’516 patents, the Federal Circuit disagreed with the district court’s finding that the inventors were not in possession of a formulation that lacked a component that is not claimed, or only optional in the claims of these patents. Id. at 22. First, the Federal Circuit reasoned the word “optional” means “something that need not be present.” Thus, the Federal Circuit found that an “optional” component does not need to be present for infringement, nor does it need to be specifically described for § 112 purposes. Id. at 23-24. Second, the specification describes two embodiments in which a glidant is not required. Id. at 24. Therefore, the Federal Circuit found that the specification as a whole shows possession through its description of a formulation without a glidant. Id. at 26.



 />i
/>i
