On April 30, 2020, the Centers for Medicare and Medicaid Services (CMS) released additional waivers and an interim final rule with comment (CMS-5531-IFC) in response to the Coronavirus (COVID-19) pandemic. These expanded flexibilities reflect the evolving needs and priorities of healthcare providers as the pandemic continues, and respond to stakeholder feedback requesting clarifications and further changes to policies from the first IFC released on March 30, 2020. The April 30 IFC also implements technical changes to help effectuate the various flexibilities.
The regulations set forth in this IFC are generally retroactive to March 1 or January 27, 2020, and are effective through the duration of the public health emergency (PHE) declaration. Comments are due on July 7, 2020.
As the PHE enters its fourth month and the pandemic continues its disruptive impact on the US healthcare system, the government has limited levers to protect providers heavily affected by COVID-19. Congress can allocate funding for direct financial relief through grants, loans or changes in tax policy, and the Administration can direct regulatory flexibilities through the issuance of regulations and waivers that protect and promote existing provider revenue streams and reduce administrative burdens. These most recent waivers and IFC represent continued efforts by CMS to help healthcare providers meet the needs of patients during this unprecedented period.
Key Takeaways
-
As hospitals and other facilities provide an increasing number of services at temporary expansion locations and experience changes in patient volume, CMS implements payment policy flexibilities and other technical changes to protect reimbursement and reduce administrative burden.
-
CMS increases payment for audio-only evaluation and management (E/M) services and proposes other changes to promote greater use of telehealth during the PHE.
-
CMS implements many changes to increase the capacity of COVID-19-related diagnostic testing throughout the duration of the PHE.
-
CMS establishes modifications to the Medicare Shared Savings Program and Medicare quality programs to support participants who are adversely affected by COVID-19.
Helpful Links
Expanded Hospital Flexibilities
CMS’s Hospital Without Walls Initiative allows hospitals to expand patient care locations during the PHE. The first IFC allowed hospitals to create surge capacity through the relaxation of various regulations, such as permitting non-hospital building space to be used for patient care and allowing currently enrolled ambulatory surgical centers to temporarily enroll as hospitals and provide hospital services.
In the latest IFC, CMS continued to support this initiative through technical changes to ensure that payment policies allow predictable Medicare payments when hospitals provide services in these temporarily expanded spaces:
-
Certain off-campus outpatient departments are paid 40% of the hospital outpatient rate. Through this IFC, on-campus departments that relocate from March 1, 2020, through the remainder of the PHE for the purposes of the pandemic may seek an exception so that they can be paid at 100% of the hospital outpatient rate.
-
Certain partial hospitalization services [1] delivered in temporary expansion locations, including patients’ homes, will be covered by Medicare (e.g., individual psychotherapy, patient education, group psychotherapy).
-
Components of certain facility payment formulas may be affected by bed capacity. CMS implemented several policies to ensure that facilities continue to receive predictable Medicare payment even if there are bed capacity changes:
-
Teaching hospitals can increase the number of temporary beds without facing reduced payments for indirect medical education.
-
Inpatient psychiatric facilities and inpatient rehabilitation facilities can admit more patients to alleviate pressure on acute-care hospital bed capacity without facing reduced teaching status payments.
-
Hospital systems that include rural health clinics can increase their bed capacity without affecting the rural health clinic’s payments.
CMS also sought to ease the administrative burden on facilities by further reducing reporting and auditing requirements. The agency supported maximizing workforce capacity through policies to allow healthcare professionals to practice at the top of their license.
These flexibilities have allowed facilities to create surge capacity during the pandemic. Many states and localities will want to maintain this ability, even with gradual re-opening, to ensure that they can increase their capacity in the event of another outbreak or hotspot development. Because these flexibilities are tied to the PHE, however, stakeholders should begin identifying longer-term changes necessary to respond to a new on-the-ground reality.
- [1] Partial hospitalization means an outpatient program specifically designed for the diagnosis or active treatment of a serious mental disorder when there is a reasonable expectation for improvement or when it is necessary to maintain a patient’s functional level and prevent relapse or full hospitalization.
Telehealth Update: Payment Increases for Audio-Only E/M Codes and Other Updates
Audio-Only E/M Codes
Expanding telehealth services is a critical component of CMS’s efforts to meet patient healthcare needs and protect healthcare providers’ exposure to COVID-19 during the PHE. In this IFC, CMS increased reimbursement for telephone E/M services (codes 99441-99443), making them equivalent to Medicare payment for office/outpatient visit codes for established patients (codes 99212-99214).
Historically, CMS has not covered audio-only E/M services. In the first IFC, CMS established separate payment for these services, but their payment rates were much less than Medicare telehealth office/outpatient visit codes:
- Prior to this IFC, payment for audio-only E/M codes ranged from $14.44 to $41.14 (office rates) and $13.35 to $39.70 (facility rates).
- Rates for the equivalent office/outpatient visit codes ranged from $46.19 to $110.43 (office rates) and $26.35 to $80.48 (facility rates).
This rate differential was based on the agency’s belief that the work billed under the audio-only E/M codes does not equate to the work billed under office/visit codes. Stakeholders urged CMS to increase the payment rate for the audio-only E/M codes because, in many cases, telephone visits are the only option for patient access. In this IFC, CMS acknowledged that during the pandemic, audio-only E/M services are “serving as a substitute for office/outpatient Medicare telehealth visits” and therefore it was appropriate to increase their payment rates.
CMS previously clarified that for Medicare Advantage providers, diagnoses captured from audio-video visits would count for risk adjustment and therefore for payment purposes in Medicare Advantage. CMS has not yet made the corresponding clarification for audio-only visits, despite the urging of providers and plans.
Other Telehealth Changes
CMS implemented several other telehealth changes, including:
-
Expanding the types of healthcare professionals that can furnish distant site telehealth services to include all providers that are eligible to bill Medicare for their professional services. As a result, a broader range of practitioners can use telehealth to provide many Medicare services.
-
Allowing certain facilities to qualify for a telehealth originating (patient) site fee. CMS will pay this fee to the facility for patients receiving telehealth services in their home or other temporary expansion site, if the beneficiary’s home or temporary expansion site is considered to be a provider-based department of the hospital, and the beneficiary is registered as an outpatient of the hospital for purposes of receiving telehealth services billed by the physician or practitioner.
-
Adding codes to the Medicare telehealth list. The list now includes more than 200 codes.
-
Using a sub-regulatory process to modify the Medicare telehealth list. Previously the rulemaking process was required to modify the list.
-
Allowing periodic assessments provided by opioid treatment programs to be furnished using audio-only technology in cases where the beneficiary does not have access to audio-visual technology.
The expansion of Medicare telehealth visits has been popular with both patients and providers. While current flexibilities are only authorized for the duration of the PHE, there is speculation that CMS will make at least some of these accommodations permanent. Stakeholders should evaluate and prioritize these flexibilities and begin to communicate to CMS and Congress about which flexibilities should become permanent.
Enhanced Access to COVID-19 Diagnostic Testing
Flexibilities regarding hospitals and telehealth show that CMS is seeking to allow providers to deliver care with minimal geographic or physical restrictions where possible. Analogously, CMS is seeking to minimize restrictions on clinicians that may supervise or order COVID-19 testing.
Supervision of Diagnostic Tests by Non-Physician Practitioners
Under existing regulations, diagnostic tests on the Medicare physician fee schedule must generally be supervised by a physician with a few specific exemptions. Non-physician practitioners may order these tests, but they may not supervise their performance. The April 30 IFC provides flexibility on this requirement by allowing non-physician practitioners to supervise diagnostic tests for COVID-19 to the extent authorized by their state scope of practice for the duration of the PHE. The extent to which this flexibility will permit additional testing therefore may vary by state.
Flexibilities Regarding Ordering COVID-19 and Respiratory Tests
Current regulations prohibit providers and suppliers from billing for laboratory tests unless the test is ordered by a treating physician and will be used to make decisions regarding the management of the patient being tested. This could limit large-scale testing and the accessibility of testing to some patients. To promote increased access to testing, CMS provided flexibilities for laboratory testing for COVID-19 and other respiratory viruses. During the PHE, COVID-19 laboratory tests and laboratory tests for other respiratory viruses may be covered when ordered by any healthcare professional authorized to do so under state law. CMS published a list of diagnostic laboratory tests subject to this waiver. Some stakeholders have requested further flexibilities regarding this kind of testing.
In line with flexibilities regarding who may order diagnostic tests for respiratory infections, CMS created flexibilities for billing for E/M visits associated with COVID-19 testing. CMS will allow the code 99211, which is usually reserved for established patients, to be billed for patients with no established relationship. To facilitate similar encounters in outpatient hospital setting, CMS created the new E/M HCPCS code C9803 (Hospital outpatient clinic visit specimen collection for severe acute respiratory syndrome coronavirus 2 (sars-cov-2) (coronavirus disease [covid-19]), any specimen source). This code will be assigned to APC 5731 Level 1 Minor Procedures, which pays roughly $23.
Coverage of Serology Tests
Serological tests that can detect antibodies are fast emerging. These may be useful in determining not only who has COVID-19, but also who has been exposed to it previously. This IFC provides Medicare coverage of US Food and Drug Administration (FDA)-authorized COVID-19 serology tests as reasonable and necessary for beneficiaries with known current or prior COVID-19 infection or suspected current or suspected past COVID-19 infection during the PHE. The IFC does not, however, set a payment rate for these tests.
Medicaid Coverage of Laboratory Tests on Self-Collected Specimens
Prior to this IFC, Medicaid only covered laboratory diagnostic tests performed in physician office settings and provided by laboratories. However, to permit flexibility for COVID-19 test coverage, including coverage for tests administered in non-office settings, this IFC allows coverage for laboratory processing of self-collected COVID-19 tests that are FDA-authorized for self-collection. The flexibility applies during the PHE and during any subsequent periods of active surveillance. While few COVID-19 tests currently allow for self-collection of the specimen, as such tests become available this flexibility will allow patients to be tested without leaving their homes or exposing specimen collection personnel to COVID-19.
The number and types of tests for COVID-19 are rapidly evolving. CMS is attempting to adapt to these developments and put processes and systems in place to increase access and enhance coverage of testing. These efforts are particularly important as state, localities, employers and employees are anxious for increased testing capacity.
Modifications to MSSP and Other Medicare Quality Programs
Entities participating in performance-based risk arrangements in the Medicare Shared Savings Program (MSSP) have expressed concerns that enduring losses could exacerbate already dire financial circumstances. Recognizing this, CMS expanded its extreme and uncontrollable circumstances policy, which limits financial losses and reductions in quality scores faced by Accountable Care Organizations (ACOs) when those ACOs or their members are affected by such circumstances. CMS has made other changes to the MSSP to attempt to prevent “windfall” savings and provide stability for participants. However, additional rulemaking will be necessary to provide additional stability for these and other value based care model participants.
Forgoing 2021 Applications, Allowing One-Year Extensions
CMS announced that it will not have an application cycle for MSSPs with a January 1, 2021, start date. Existing ACOs whose agreements end on December 31, 2020, will have the option to extend their participation agreements effective January 1, 2021. In announcing this change, CMS cited two primary reasons: to allow entities to focus on their patients during the pandemic rather than completing applications, and to give the agency more time to consider and develop strategies to address the impact of 2020 on future benchmarking.
CMS redesigned the MSSP under the Pathways to Success final rule in 2018. Under the final rule, CMS finalized the BASIC track, which allows MSSPs to incrementally phase in higher levels of financial risk and reward along a glide path with five levels, A through E. ACOs are automatically advanced a level each year, unless the ACO elects to advance more quickly.
CMS indicated in the IFC that it will allow ACOs in the BASIC track to elect to maintain their current level of risk for 2021. For 2022, these ACOs will advance two levels (bypassing the level they skipped in 2021). For example, a Level B ACO that stays in B for 2021 will participate in Level D in 2022.
Extreme and Uncontrollable Circumstances Policy Linked to the PHE
The extreme and uncontrollable circumstances policy attempts to provide downside protection for MSSP ACO’s Under this existing policy, CMS reduces the amount of the MSSP ACO’s shared losses and quality performance to account for the total months and percentage of beneficiaries affected by the extreme and uncontrollable circumstances.
CMS recognized in the first IFC that 100 percent of MSSP assigned beneficiaries reside in an area affected by COVID-19. In the April 30 IFC, CMS clarified that the months affected by the extreme and uncontrollable circumstances policy will begin with January 2020 and continue through the end of the PHE, including any subsequent PHE renewals. Therefore, if the PHE extends through the end of 2020, the policy would mitigate against all shared losses. However, if the PHE runs for only six months, losses would be reduced by half. The current PHE extension runs 90 days from April 21, 2020. We expect that the PHE will be renewed in July, since broad flexibilities are still necessary to respond to the virus as states continue the work of re-opening. Therefore, while this policy provides some protection against shared losses for MSSPs, some level of uncertainty remains after October.
Accounting for COVID-19 in the MSSP Financial Model
The Coronavirus Aid, Relief and Economic Security Act specified that for COVID-19 discharges, the Secretary of the US Department of Health and Human Services would increase the weighting factor applied to the Diagnosis Related Group (DRG) by 20%. To account for the impact of this increased payment and associated costs preceding and following hospitalization, CMS will exclude all Part A and B fee-for-service payment amounts for an episode of care for treatment of COVID-19 triggered by a qualifying inpatient service. CMS will define the episode of care as starting in the month in which the inpatient stay begins, all months of the inpatient stay, and the month following the end of the inpatient stay. CMS will use its authority to adjust benchmark expenditures for other factors in order to remove these COVID-19 related expenditures from the determination of benchmark expenditures and other program calculations, including determination of performance year expenditures.
It may be difficult at this time for ACOs to model this policy change and therefore to determine the full impact of this adjustment. ACOs also will come to see the impact of COVID-19 on their benchmarks and performance over the months and possibly years to come. CMS likely will need to make additional adjustments to protect entities against the impact of COVID-19.
Adjusting Beneficiary Alignment for the Shift to Virtual Visits
In the MSSP, CMS assigns beneficiaries to ACOs based on each beneficiary’s utilization of primary care services provided by ACO physicians. CMS revised the definition of primary care services used in the MSSP assignment methodology for the January 1, 2021, performance year and subsequent performance years that start during the PHE to include certain telehealth and telemedicine services. Given the widespread use of virtual care services during the PHE, ACOs should consider whether additional modification to this policy may be necessary to prevent certain patients from being swept into their alignment who should not be aligned.
Quality Program Changes
CMS made several modifications to other Medicare quality programs.
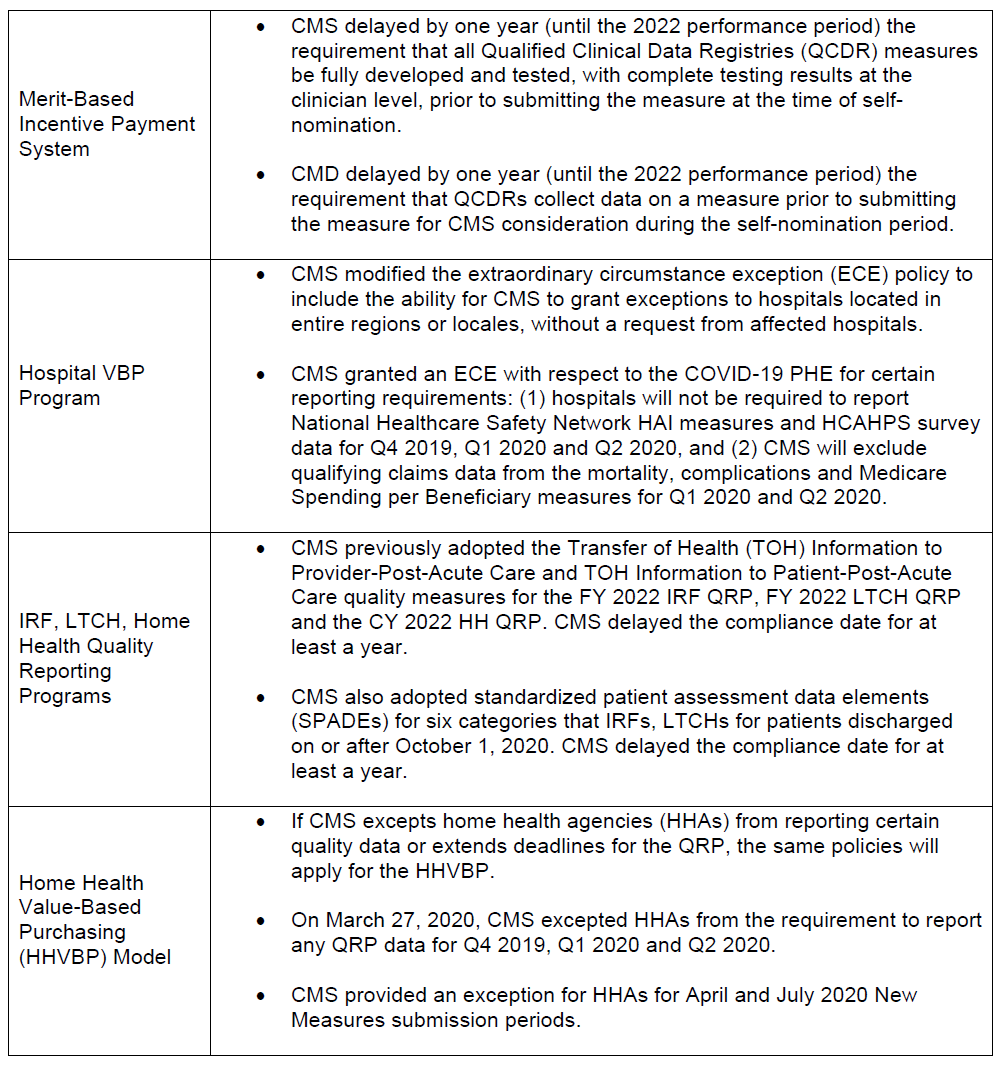
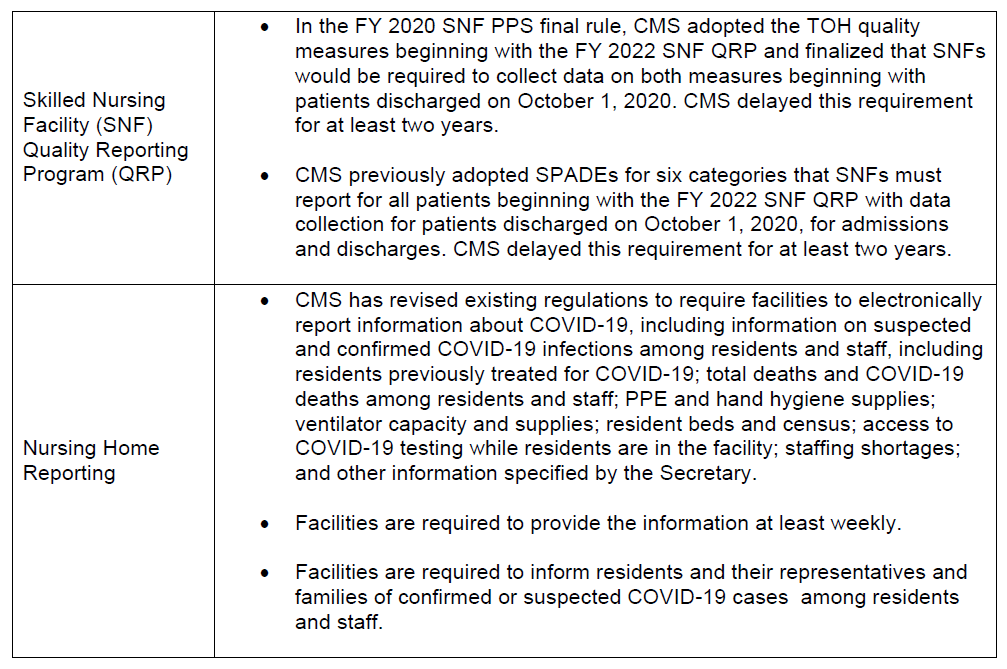
What’s Next
The policies announced by CMS through the IFCs and waivers are currently tied to the length of the PHE, which currently is set to expire at the end of July 2020. The Secretary can renew the PHE for 90 days and has already done so once. As the pandemic has continued to spread, the challenges facing healthcare systems have evolved. The first IFC introduced sweeping flexibilities to help facilitate the provision of services in the challenging environment created by COVID-19. This second IFC, while perhaps less sweeping and more technical in nature, reflects the evolving needs and priorities of healthcare providers, clarifies and revises previous policies, and incorporates stakeholder feedback.
Today, states and localities are phasing into a re-opening or providing guidance on how and when to do so. This shift may create fresh challenges for healthcare systems and providers across United States. For example, flexibilities concerning inpatient rehabilitation facilities are tied to the White House “Opening Up America Again” guidelines, which may mean that flexibilities apply in different ways in different parts of the country going forward. This could present both adherence and enforcement challenges to Medicare contractors overseeing locales that are under different phases of these guidelines.
Because CMS has limited tools at its disposal to address the pandemic, it is conceivable that the agency will release another IFC in the future to address these and other issues. Stakeholders have an opportunity to provide input to the agency on their needs for future flexibilities through the comment process for this current IFC.
Stakeholders should also reach out to CMS outside of the comment process and make a case for their needs and priorities. In some areas, such as telehealth policy, CMS has moved incrementally, by making a change, then revisiting the issue and making broader changes. This approach suggests that CMS is willing to further adapt policies to meet the evolving needs of healthcare providers.





 />i
/>i

